International Research Journal of Engineering and Technology (IRJET)

Volume: 09 Issue: 05 | May 2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072
EMPHASISE OF STEEL REINFORCEMENT IN CONCRETE AND GEOPOLYMER COATINGS FOR CORROSION PROTECTION OF STEEL REINFORCEMENT IN CONCRETE(SRC)
N. CHANDRIKA1 , T. PRAMOD KUMAR2
1PG Student, Department of Civil Engineering, Nova College of Engineering and Technology, Affiliated to JNTU Kakinada, Vegavaram, Jangareddygudem, West Godavari (District), A.P 534447.
2Guide, Assistant Professor, Department of Civil Engineering, Nova College of Engineering and Technology, Affiliated to JNTU Kakinada, Vegavaram, Jangareddygudem, West Godavari (District), A.P 534447
Abstract - Generally, In construction the industries are facinga corrosioninsteel. Thesteelinreinforcedconcretehas been found as the significant factor leadingprematurefailure of concrete constructions. Durability of concrete structure is placed as main global issue in construction sector. The loss of durability and life of construction is directly depend on corrosion of steel. A lot a research as done to control corrosion in steel and to prevent, among that application of coating to steel reinforcement is an effective method of corrosion control. These two mechanisms must be controlled while coating on steel (i) Chloride Attack and (ii) Carbonation of Concrete. The coated steel is impressed in water and chlorides and its very tuff to achieve perfect epoxy coating. In the circumstance of galvanized steel reinforcement, the connection ofuncoated steel rebars with coated steel bars should be evaded to realize the long term corrosion defense presentation of galvanized steel rebar. The epoxy coated reinforcement increases permeability between the concrete and decreases corrosion in reinforcement against which is not coated reinforcement. To improve the performance of the coating reinforcement should be focused on their basic characteristics of proper resistance to water, adhesion, chemical, abrasion, frictionandcost. The present work focused on improving long period corrosion for steel. Based on collection of literature, the geopolymer coating for steel have many advantages and having excellent controlling corrosion and fire resistance properties, Improvinga goodbondstrength between coatingofsteeland concrete. By knowing advantages from literature collection, the investigationhas done offeringbygeopolymerbinder.The two main constituents of geopolymer places a role in this investigation. Such that investigation source are mainly materials and alkaline liquids. The selected source materials are; by product materials such as fly ash, Ordinary Portland Cement(OPC), Microsilica, Rice husk ash, Kaolin, Ferrosilicon Powder, Vanadium Pentoxide and Silica fume were used as reactive material, combination of Sodium Hydroxide (NaOH) and Sodium Silicate (Na2SiO3) as alkaline liquid used for geopolymerisation. Ten formulations were made and experimental evaluation has been carried out on all the studied coatings for its adhesive strength, flexibility, bendability, corrosion resistance, saltspray, impact test.
***
1. INTRODUCTION
Corrosion ofsteel in concrete involves a complex series of reactions, the proportions of which may vary with environmental exposureandmaterialscharacteristics.Ina high pHenvironment,suchasconcrete,hydroxide(OH)is abundantinsolution. Hydroxideionsmayreactwithironat the steel surface to create ferrous hydroxide (Fe (OH)2). Carbonationisaprocessin which carbon dioxide diffuses intotheconcretefromtheair.Thereactionproduct, calcium carbonate,has a pH of about 9.5, which is below the level necessarytosustainthepassivelayer
[Ca(OH)2+CO2CaCO3 +H 2O] 1.1.
[Calciumsilicatehydrates+CO2CaCO3 +SiO2nH 2O+H 2O] 1.2.
[Aluminatehydrates+CO2CaCO3 +hydratedalumina] 1.3.
[Ferritehydrates+CO2CaCO3 +hydratedaluminaandiron oxides] 1.4.
[Anode:3Fe+8OHFe3O4 +4H2O+8e] 1.5.
[Cathode:8e+4H2O+2O28OH] 1.6.
1.1 Geopolymer Coatings
Geopolymer is an inorganic polymer a alkali activated binder) has gained worldwide interestand its high anticorrosionpropertymadewithacoating materialand it has the benefit of fire resistance.Thereactionofsolid aluminosilicate material with highly silicate solution produceasyntheticalakalialuminosilicate,thusproduced material generically called “geopolymer” but in more of referencesthattheytermedasan“Inorganicpolymer”.The geopolymer solution can be tailored by correct mix and processing to optimise the properties such as flexibility, adhesionandtoofferexcellentcorrosionresistancecoatings withreducedcost.
IRJET | Impact Factor value: 7.529 | ISO 9001:2008
International Research Journal of Engineering and Technology (IRJET)

Volume: 09 Issue: 05 | May 2022 www.irjet.net
1.2 Geopolymer Processing
In general, the reactive alkali solution is prepared with sodiumhydroxide(NaOH)andsodiumsilicate(Na2SiO3)and the alumina silicate solid powders can be of fly ash, microsilica, kaolin, clay, high alumina clay etc. in which sufficientamountofsilicate(SiO2)andalumina(Al2O3)must bepresent.Duringthereactionofaluminasilicatematerials withthe alkalibindersolution,waterisgraduallysplitout andthetetrahedralunits(SiO4andAlO4)alternativelylinked to polymeric precursors ( SiO4 AlO4 (or) SiO4 Al2O4 SiO4 (or) SiO4 AlO4 SiO4 ) by sharing oxygen atoms forming amorphousgeopolymericproductswiththree dimensional networkstructure
2. Alkaline Liquids and Source Materials
Inthepresentexperimentalwork, therearedoublemain elements of geopolymers, viz. The source materials for geopolymersgroundedonalumino silicatemustbeina mannerofrich,thenthesilicon(Si)andaluminium(Al). Thesecouldbenaturalmineralssuchaskaolinite,clays, micas,spinel,etc.whoseempiricalformulacontainsSi,Al, andoxygen(O). Alternatively,theby productmaterials such as fly ash, Microsilica, Rice husk ash, Eluru Clay, Kaolin,Ferrosilicon Powder, and Silica fume wereused as a filler material. The high quality of the cause suppliesformakinggeopolymersrestonfactorssuchas reactivity availability, cost and type of submission and specificrequestoftheendusers.Thealkalineliquidsare from soluble alkali metals that are usually Sodium or Potassiumbased.Materialsrichinsilicon(suchasflyash orslag)andmaterialsrichinaluminum(kaolinclay)are theprimaryrequirementforgeopolymerizationtooccur.
The details of the sources for the raw materials and their chemical compositionsaregiveninTables1and2
Table 1: ProcurementdetailoftheRawMaterials
Name of the Material Procurement Details
Flyash CollectedfromKrishnaBabu
PalGBricks,Eluru,India
Microsilica PurchasedfromIndiaMart
Ferrosilicon PurchasedfromIndiaMart
EluruClay Procuredfromlocalchemicalssuppliers.
Ricehuskash Procuredfromlocalricemill.
Clay Collectedfromnearbyponds.
Chemicals used
e ISSN: 2395 0056
p ISSN: 2395 0072
Kaolin PurchasedfromIndiaMart
Gradedfine sand Screenedriversandwithfineness modulusequalto2.58conformingto gradingzoneIIIofIS:383,1970.
Table 2: ChemicalCompositionofMineralConstituents byXRF(wt%)
Material used
SiO2 wt% Al2O3 wt% Fe2O3 wt% MgO wt% CaO wt% Density g/cm3
OPC 21.0 6.04 3.79 2.6 63.5 3.4
FlyAsh 44.3 23.5 11.75 1.3 18.3 2.6
Micro silica 97.5 0.75 0.64 0.4 0.65 2.7
Ferrosilic on 78.2 0.46 11.40 1.1 1.21 2.2
Eluru clay 68.0 21.0 0.05 8.34 1.9
Ricehusk Ash 94.0 0.26 0.18 0.4 0.67 2.1 Clay 65.5 15.1 11.78 0.7 1.38 1.8 Kaolin 46.5 39.5 1.55 2.6
2.1 Preparation of ten Geopolymer Coatings
NaCl,NaOH,S2Cl2 Sb2O3,HCl,Na2SiO3 wereofARgrade.Solutionswere preparedwithdoubledistilledwater.
Geopolymerbinderpropertiesarehighlydependentuponthe type,ratiosandconcentrationsofmixingconstituents.Each constituentandthevariablesassociatedwiththatconstituent playasignificantrole indeterminingthecharacteristicsofthe final product. The resulting geopolymer solution (binder solution)wasin1:3ratio,onepartofNaOHandthreepartsof Na2SiO3(120 gm Na2SiO3) Mix thoroughly still the sodium silicatesolutionfullydissolvedinNaOHsolutionThisbinder solutionwasusedtopreparethefinalcoatingformulationas describedinTable2 Thevolumeofthebindersolutionand reactive material indicatedintheabovetableaftertrialand errorhasresultedthebrushablecoating, driedin24hoursat room temperature with acceptable adhesion. The prepared geopolymer binder was mixed with required quantity and combinations of fly ash, microsilica, OPC, Kaolin, clay, Eluru clay,ferrosiliconpowder,ricehuskashandVanadium Pentoxide (V2O5) in such a way that the resultant solution givesbrushableconsistencyandoffersroughsurfacefinishin ordertoenhancethebondingbetweenthecoatedsteeland theconcrete.Twocoatswereappliedtoavoidtheformationof pinholes in the coatings. The formulated coatings were brushed two coats to get uniform film thickness. All the experimentswerecarriedoutatanambienttemperatureof32 ± 1º C. Then in the second day, it was kept in an oven at 50º for threehourstoacceleratethecrosslinkingandlaterit wasallowedtocurefor5daysintheambientcondition.The pigment and fillers were selected to react with geopolymer solutiontoform
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified
International Research Journal of Engineering and Technology (IRJET)

Volume: 09 Issue: 05 | May 2022 www.irjet.net
Sl.No Coating Code Materials used
1 GPR Binder(25ml)+Flyash(10g)+IronoxideRed (2g)+Silicafume(0.2g)
2 GP1 Binder(30ml)+Flyash(10g)+Ironoxide yellow(2g)+Silicafume(0.2g)

3 GP2 Binder(30ml)+Microsilica(10g)+Ironoxide yellow(2g)+Silicafume(0.2g)
4 GP3 Binder(30ml)+Flyash(5g)+Microsilica(5g) +Ironoxideyellow(2g)+Silicafume(0.2g)
5 GP4 Binder(50ml)+OPC(10g)+Ironoxide yellow+Silicafume(0.2g)
6 GP5
Binder(40ml)+OPC(5g)+Flyash(5g)+Iron oxideyellow(2g)+Silicafume(0.2g)
7 GP6 Binder(40ml)+OPC(5g)+Microsilica(5g)+ Ironoxideyellow(2g)+Silicafume(0.2g)
8 GP7 Binder(40ml)+OPC(3g)+Flyash(4g)+ Microsilica(3g)+Ironoxideyellow(2g))+ Silicafume(0.2g)
9 GP8
Binder(40ml)+OPC(5g)+Kaolin(5g)+Iron oxideyellow(2g)+Silicafume(0.2g)
10 GP9 Binder(50ml)+Flyash(5g)+Kaolin(5g)+ Ironoxideyellow(2g)+Silicafume(0.2g)
Table 2:Nomenclaturefordifferentcoatingsystems
e ISSN: 2395 0056
p ISSN: 2395 0072
Fig-4: Elcometer456CoatingThicknessGaugeDevice
TheemployedElcometer456CoatingThicknessGaugewas showninFigure3Itprovidesreliableandaccuratecoating thicknessmeasurements.Theprincipleofelectromagnetic induction is used for non magnetic coatings on magnetic substratessuchassteel.


3. RESULTS AND DISCUSSION
3.1 Coating Thickness

The thickness of coating was measured using a handy digital display thickness measuring Elcometer 456 as shown in Figure 4 Upon each coated surface, 6 measurements are averaged and the average values are reported in Table 3 This coating thickness is called dry filmthickness(dft)andwasgiveninmicrons.Theobtained values lie in the range of 210µm to 237µm.The coatings
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified
International Research Journal of Engineering and Technology (IRJET)

Volume: 09 Issue: 05 | May 2022 www.irjet.net
wereappliedwithbrush.Twocoatsweregivenonpickled andcleanedsurfaceofthemildsteelpanels.
Designation of Coating
Coating Thickness (urn)
Load at Failure N
Adhesion Test (by Tensometer) Flexibility Test (ASTMD 522)
Stress at Failure N/mm2
Impact Test
On coated plates ASTMD 2794
On coated rods ASTM D14
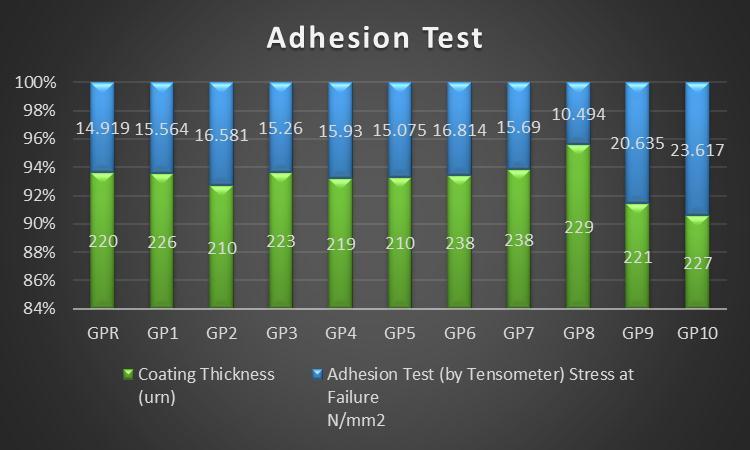
GPR 220 7.585 14.919 P P P
GP1 226 8.122 15.564 P P P
GP2 210 8.831 16.581 P P P
GP3 223 7.892 15.260 P P P
GP4 219 7.746 15.93 P P P
GP5 210 7.961 15.075 P P P
GP6 238 8.814 16.814 P P P
GP7 238 8.213 15.690 F P P
GP8 229 5.171 10.494 P F F
GP9 221 10.150 20.635 P P P
GP10 227 12.123 23.617 P P P
Table 3: ResultsofMechanicalPropertiesofCoatings
4. SUMMARY
Allthecoatedplateswithscribedononesideandtheother side as unscribed are edge sealed using wax and were exposed to open atmosphereforaperiodof60days(1400 hours).Fourtimesaday,3%NaClwassprayedontheplates andobservationwasmadeinrespectofcorrosion. Except thecoatingGPRshowsthemarkofcreepageandremaining allothercoatingsshowednomarkofcreepageandtherefore nofailureof coatings due to corrosioncreepage.Thiswas accreditedtothe excellentadhesionstrengthbetweenthe steel plate and the coating. However, the coatings GPR earned the rating of 4 respectively at theendof60days‟ exposure.
e ISSN: 2395 0056
p ISSN: 2395 0072
Geopolymer is an inorganic polymer, an alkali activated binderwhich has gained worldwide interest and its high anticorrosionpropertymadeitanovelcoatingmaterial The reaction of solid aluminosilicate material with highly concentrated aqueous alkali hydroxide or silicate solution produce a synthetic alkali aluminosilicate material generically called “geopolymer” and can be compared in performance with the traditional cementitious folders ina rangeofapplications,butwiththesupplementarybenefitof suggestivelyconcentratedgreenhouse releases.
The geopolymer solution can be tailored by correctmix and processing to optimise properties such as flexibility, adhesion and to offer excellent corrosion resistance propertieswithreducedcostforgivencoatingapplications. Therefore, by considering the advantages offered by geopolymerbindersuchasgreenermaterial,goodcorrosion andalkali, acidresistance,fireresistanceandalsoexcellent adhesion to steel substrate with high electrical insulating effects,thegeopolymerbinderischosenwithdifferentkind ofaluminosilicatematerials.
Tendifferentgeopolymerbasedcompositionsusingflyash, OPC, Microsilica, Rice husk ash, Clay, Eluru Clay, Kaolin, FerrosiliconPowder, Vanadium Pentoxide, Silica fume and Fe2O3 asfunctionalpigmentsandfillerswereformulatedand brush able coating materials were synthesised. The geopolymersolution(bindersolution)waspreparedin1:3 ratio,ofNaOHandNa2SiO3 andmixedthoroughly.
5. CONCLUSION AND FUTURE WORK
A systematic study on all the prepared ten geopolymer coatingsresultedthefollowingobservations.
The average thickness of the studied coating varies from 210µm to 238µm.Coating thickness above210µmhasnorole in performanceonlythe ingredientshasmainroleinprotection.
GP16showedpooradhesionstrengthperhapsdue to the presence of ferrosilicon and poor cross link formation. The multiple hydroxyl group in GP12 developed good adhesion withthe steel plate and chemical anchoring in addition to mechanical anchoringofcoating.
The presence of rice husk ash in the coating formulation improved the drying and thus results tight bonding between the steel plate and coating. GP7 and GP8 8.213 N/mm2 were failed at 3mm diameteroftheconeandpassedat6mmdiameterof thesamecone.Furtheralltheothercoatingswere foundtopossessverygoodflexibilitycharacteristics. Theadditives such as microsilica,ricehuskashdo not possess required amount of aluminium oxide
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page176
Chart 1: Adhesionstrengthdataoftencombinationsof GeopolymercoatedplatesresultedfromAdhesiontestInternational Research Journal of Engineering and Technology (IRJET)

Volume: 09 Issue: 05 | May 2022 www.irjet.net
(Al2O3) for the dense cross linking and therefore poorflexibility.
Also, during film formation, evaporation of water fromthefilmleftswithmicrovoidsandthatreduces thestrengthagainstflexibility.
Pencilhardnesstesthasbeencarriedoutonallthe coatedpanelsandtheresultssuggestedthatallthe studiedcoatingswerepassedinthistest.
The coating GP9 and GP10 performed better in all thetestproducesincludingelectrochemicaltests.
Field exposure studies also give GP9 and GP10 as goodcoatings.
Inelectrochemicalimpedancespectroscopytest,the coatings GP7,GP8,GP9and GP10werefoundhigh resistance coatingeven after 30 days of periodical test, this is due to chemical and mechanical anchoringofthecoatingwithplates.
GP8, GP9 and GP10 have performed excellently under salt spray test conducted for a period of 60 days(1400hours).Theperformance was attributed to the strongadhesion of betweentheplateandthe coating.
In conclusion, GP9 and GP10 have passed all the examinationsaspertheexperimentalconditionsadopted in the present study and may be very well utilized for preventing or decelerating the corrosion rate of steel rebarsinconcrete.
REFERENCES
1. Balaguru,PN,Nazier,M&Arafa,M2008,„Field ImplementationofGeopolymerCoatings‟,Dept.of CivilandEnvironmentalEng.
2. Bavidovts, J 1991, J. Therm. Anal, vol. 37, p. 1693.
3. Bentur,A,Diamond, S&Berke,N1997,„Steel CorrosioninConcrete‟,Chapman&Hall,London.
4. Broomfield&John,P2007,„CorrosionofSteel in Concrete: Understanding‟, Investigation and Repair.Taylor&Francis(SecondEdition).Byfors& Kajsa 1986, „Chloride binding in cement paste‟, NordicConcreteResearch,vol.5,pp.27 38.
5. Chareerat, T, Lee Anansaksiri, A & Chindaprasirt,P2006,„Synthesis ofHighCalcium FlyAshandCalcinedKaolineGeopolymerMortar‟, InternationalConferenceonPozzolan,Concreteand Geopolymer,KhhonKaen,Thailand,pp.24 25.
e ISSN: 2395 0056
p ISSN: 2395 0072
6. Cheng, TW & Chiu, JP 2003, „Fire resistant geopolymerproducedbygranulatedblastfurnace slag‟,MineralsEngineering,vol. 16, no.3,pp.205 210.
7. Comrie, DC, Paterson, JH & Ritcey, DJ 1988, „Geopolymer Technologies in Toxic Waste Management‟Proceedingsofthe1stInternational ConferenceonGeopolymer,Compiegne,France,vol. 1,pp.107 123.
8. Davidovits,J1988d,„GeopolymericReactionsin Archaeological Cements and in Modern Blended Cements‟,PaperpresentedattheGeopolymer‟88, First European Conference on Soft Mineralurgy‟, Compiegne,France.
9. Davidovits,J1994,J.Mater.Educ.,vol.16,p.91.
10. Davidovits,J1999,„ChemistryofGeopolymeric Systems, Terminology‟, Paper presented at the Geopolymere‟99International Conference, Saint Quentin,France.
11. Davidovits, J 2008, „Geopolymer Chemistry and Applications‟, Institute Geopolymere: St. Quentin,France.
12. Davidovits, J 1988b, „Geopolymer Chemistry andProperties‟,ProceedingsoftheFirstEuropean ConferenceonSoftMineralurgy,Compiegne,France, p.88.
13. Davidovits, J 1988a, „Soft Mineralurgy and Geopolymers‟,Proceedingsofthe1stInternational Confer Conference on Geopolymer, Compiegne, France,vol.1,p.19 23.
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008
