International Research Journal of Engineering and Technology (IRJET)

Volume: 09 Issue: 04 | Apr 2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072

International Research Journal of Engineering and Technology (IRJET)

Volume: 09 Issue: 04 | Apr 2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072
aM.Tech Scholar, Department of Mechanical Engineering, Sri Sai College of Engineering & Technology, Badhani
bAssistant Professor,Department of Mechanical Engineering Sri Sai College of Engineering & Technology, Badhani
Abstract: The multiple principal elements containing HEAs generally contain 5 13 elements with atomic percentage in between 5 and 35 for each element. The minimum 5 elements are taken to get enough entropy to avoid the intermetallic formationandsegregationofelements.Themaximum13elementsaretakenbecausebeyondthislimit,thereisnotsignificant contributiontoentropyhoweverthecomplexityincreases.Inadditiontothemultipleelements,someminorelementscanalso be added with atomic % below 5%. The current research concerns with the characterization and corrosion performance of highentropyalloys.Forthe preparationofHEAs, meltingandcastingroute wasadopted byconverting thegreencompacted pellets in to button shaped final samples. All the samples were characterized using X ray diffraction (XRD) and optical microscopy.Also,thehardnessofthesampleswasassessed.Thecorrosionbehaviorofthesampleswasinvestigatedusingthe potentiostatin3.5%NaClsolution.
Keywords: High entropy alloys, Electrochemical Corrosion, Corrosion current density, Corrosion potential
1. INTRODUCTION: The practice of alloying has been steadily improving since the Bronze Age, with significant improvementsalongtheway.Ithaslatelybeenapopularstudytopictofocusonimprovingrevolutionarymulti elementalloys in order to fulfill the needs of sophisticated applications that require greater performance. Traditional alloys are primarily comprised of one or two basic elements that have been selected to meet a particular property need for a certain use, with furtheralloyingadditionsimprovingthosecapabilities.High entropyalloys(HEAs),ontheotherhand,arearelativelyrecent idea in alloy design that comprises five or more main elements, each of which is mixed individually before being combined together.TheinvestigationofHEAsbeganin2004withthecompletionoftwoseparateinvestigations.Cantoretal.[2]andYeh etal.[3]bothtooktheinitiativetodealwithalloysinthecenterofthephasediagramratherthantowardstheapexesor edges of the phase diagram, respectively. In a successful synthesis of an equi atomic FeCrMnCoNi alloy with a single FCC phase, Cantoretal.[2]demonstratedthepresenceofdendritic(havingalmostequalproportionsofeachelement)andinter dendritic (low in Fe but rich in Cr and Mn) regions in the microstructure, as well as the presence of dendritic (having almost equal proportionsofeachelement)regions.Whenthesixthelement(Nb,Ti,V,Cu,Ge,onebyone)wasintroducedintothisalloy, it was discovered that the FCC phase of FeCrMnCoNi was capable of dissolving significant amounts of Nb, Ti, and V but not significantamountsofCrandGeduetothehigherelectronegativityofthelattertwoelements.Forthefirsttime,Yehand his colleagues[3]publishedwell explainedresearchonmulti componentalloysthatwasfavorablyreceived.Yehdesignatedthese alloys as 'High Entropy Alloys (HEAs)' because he believed that the entropy of mixing for equimolar alloys containing more thanfivemajorelementswouldbeadequatetoreducethepropensityoforderingandsegregation,asaresultofwhichsimple phaseswouldoccur.Inhisdefinition,highentropyalloysareequi molaralloyscontainingfiveormoreprimarycomponentsin equalproportions.Addingboron(B)toCuCoNiCrAl0.5FeHEA(Yeh'sHEA)resultedinasingleFCCphase,asvalidatedbyXRD analysis,whichconfirmeda singleFCCphaseforallCuCoNiCrAl0.5FeBxHEAs,inwhichpeakintensitiesofCrandFeborides emergedandrosebyincreasingthequantityofB,respectively.
A number of Mn containing high strength and/or high ductility HEAs have been described [4, 5, 6, and 7], although the majorityofthemwouldhavelimitedanticorrosiveperformanceduetotheirMncontent.Severalinvestigationsonthecreation ofinnovativeHEAs[8 13]haveshownthatstrikingabalancebetweencorrosionresistanceandmechanicalqualitieshasbeen difficult to accomplish in earlier studies on this topic. The alloy AlxCoCrFeNi (x = 0.3, 0.5, and 0.7) had high corrosion resistance in 3.5 weight percent NaCl [13], but it exhibited low ductility owing to the brittle BCC eutectic phase in the alloy [14].Similarly,inthecaseofMo dopedFeNiCoCralloys[24],asimilarsituationhasbeendescribed.Furthermore,ithasbeen shown that the alloying of Cu in the FeCoNiCrCux system (x = 0, 0.5, and 1) not only accelerates localized corrosion but also degradesplasticityduetothecreationofaCu richinter dendriticphase[12].Infact,forstructuralmaterialsthatareusedin
Impact Factor value: 7.529
9001:2008
International Research Journal of Engineering and Technology (IRJET)

Volume: 09 Issue: 04 | Apr 2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072
severeenvironments,agoodmixofmechanicalcharacteristicsandanti corrosionperformanceisrequired[14].Consequently, itiscriticaltoinvestigatecorrosion resistantalloysthathaveoutstandingmechanicalproperties.
Al,Co,Cr,Cu,Fe,Ni,andTipowderswereusedasrawmaterialsforthepresentwork.Table1showsthecompositionofHEAs investigatedinthepresentstudy.
Alloy Al Co Cr Cu Fe Ni Ti
HEA1 4 18 18 2 18 35 5
HEA2 4 18 18 2 18 33 7
HEA3 4 18 18 2 18 31 9
HEA4 4 18 18 2 18 30 10
The powders as per the compositions were blended and converted into cylindrical pellet using 15 mm die by applying 120 MPapressure.TheHEAsweremadeinaninertenvironmentusingthearcmeltingprocedure.Thealloyswerethenanalysed, andtheHEAs'corrosionbehaviourinNaClsolutionwasexamined.Themeltingandflippingofthesampleswereperformed5 timesforhomogeneousstructure.
3.1 X Ray Diffraction, hardness and optical microscopy
X raypowderdiffraction(XRD)isarapidanalyticaltechniqueusedforphaseidentificationofmaterials.Thephaseanalysisof HEAs was done by X Ray Diffraction (XRD) 30° to 90° using Cu Kα radiation. The peaks obtained from XRD plot of powders were fitted through High Score Plus (Version3.0d) using pseudo Voigt function. After peak fitting of all XRD peaks, peak positionandthenlaterlatticeparameterwascalculated.Thehardnessofall theHEAswasestimatedusingVickershardness tester.Theopticalmicrographsweretakenforallthesamplesusingopticalmicroscope.
ThecorrosionbehaviourofalloyswasinvestigatedusingaPotentiostatin3.5wt%NaClsolution.Thecorrosionbehaviourof materialswasstudiedusingathree electrodecellwithreference(SCE),counter,andworkingelectrodes(HEAs).Theicorrand Ecorr values were found out by fitting the curves. These values were found by fitting the polarization curves. Then, the corrosionrateisdeterminedbytheformula[10]:
CR dA
where Icorr is the corrosion current in amperes and calculated using the Tafel extrapolation method where the cathodic reactionisdiffusioncontrolled,Kisaconstantequalto3.27×103;EWisequivalentweight.
Figure1showstheXRDpatternforHEA1,indicatingtheformationofmajoritysignificantpeaksofFCCphase.Similarly,HEA2 showed mainly single FCC phase as shown in Figure 2. The XRD plots for HEA3 (Figure 3) and HEA4 (Figure 4) are almost similar.ThesignificantFCCpeaksare(111),(200),and(220).
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified
International Research Journal of Engineering and Technology (IRJET)

Volume: 09 Issue: 04 | Apr 2022 www.irjet.net
Figure3XRDPlotofHEA3.
e ISSN: 2395 0056
p ISSN: 2395 0072
Figure4XRDPlotofHEA4.
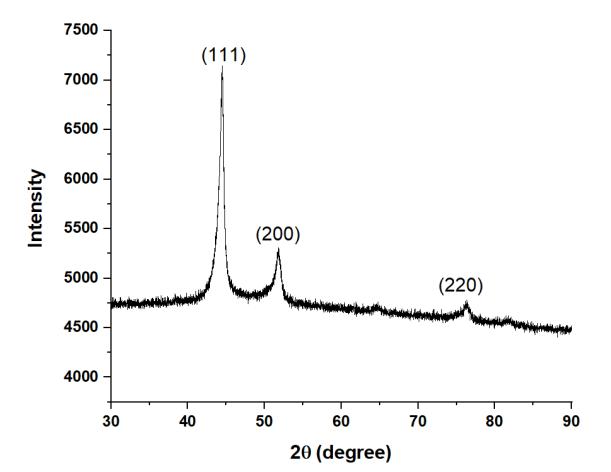
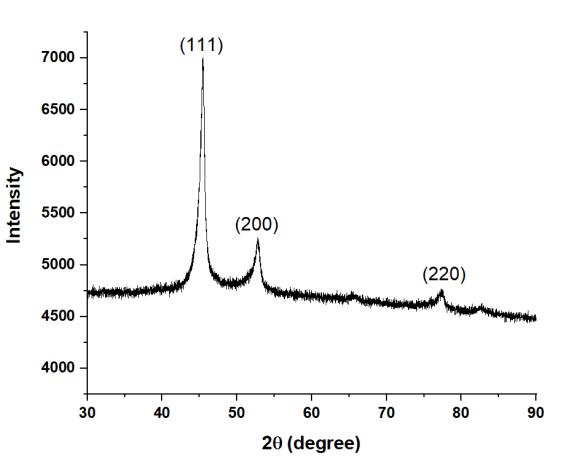
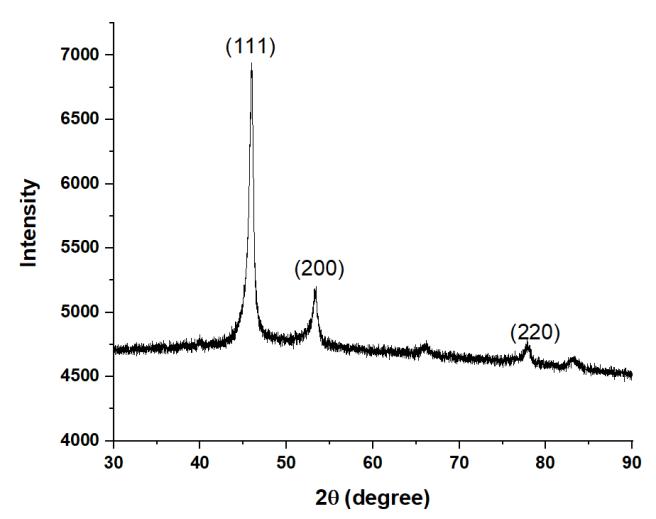
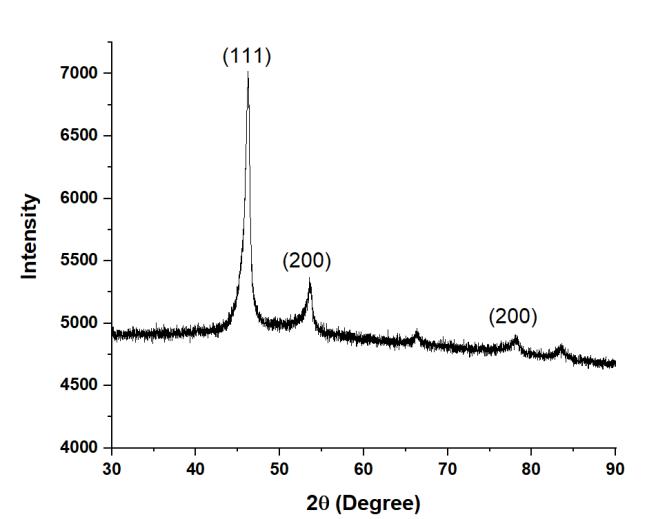
Figure5showstheleftwardshiftofthemostprominentXRDpeakfromHEA1toHEA4.Itindicatesthatthelatticeparameter isincreasingduetolargeatomicradiusofTi.ThisextensionoflatticeparametercanbeclearlyseeninTable2.
IRJET | Impact Factor value: 7.529 | ISO 9001:2008
International Research Journal of Engineering and Technology (IRJET)

Volume: 09 Issue: 04 | Apr 2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072
Table2LatticeparameterofHEAs
HEA Latticeparameter(A)
HEA1 3.559
HEA2 3.567
HEA3 3.582 HEA4 3.587
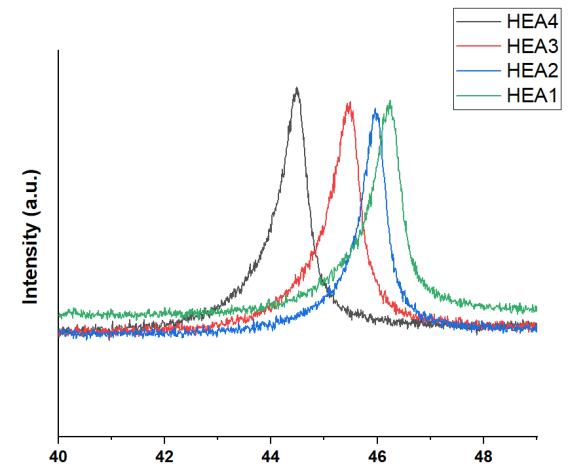
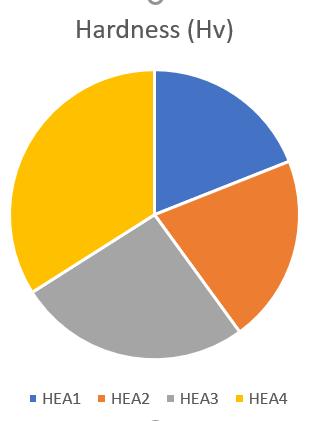
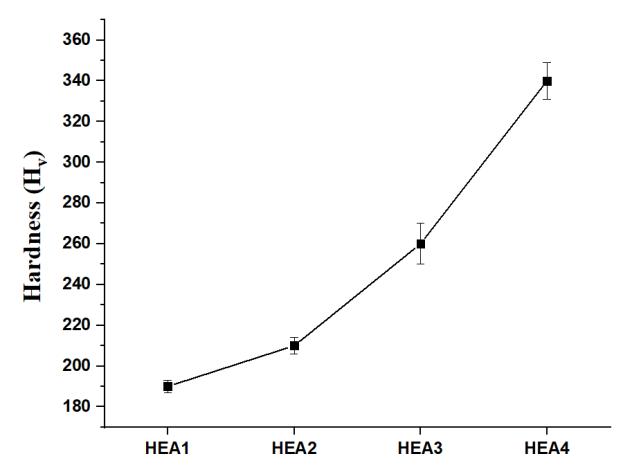
Figure 6 and Table 3 reveal the hardness of all HEAs. The percentage of Ti in alloy enhanced its hardness due to its large atomic size andimpact of solid solution strengthening.As a consequence, Ti acts as a solid solution strengthener, increasing thehardnessandstrengthofalloys.SimilarcontributionofTitohardnesshasbeenobservedinliterature[15],inwhichtheTi additiontoCoCrFeNiAl0.5increasedthehardnessvalue.
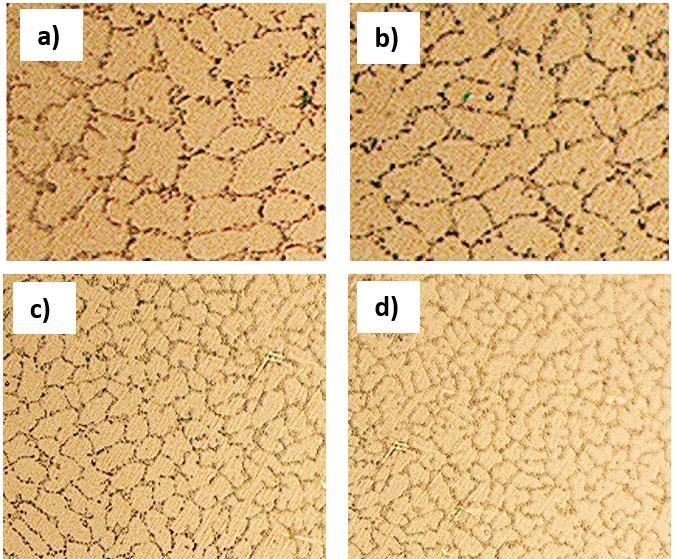
TheopticalmicrographsweretakenoftheannealedHEAsasshowninFigure 8.ThegrainsgotrefinedwiththeincreaseofTi content in HEAs because Ti might have hindered the grain growth due to high melting point and precipitations at grain boundaries.
Impact Factor value: 7.529
9001:2008
International Research Journal of Engineering and Technology (IRJET)

Volume: 09 Issue: 04 | Apr 2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072
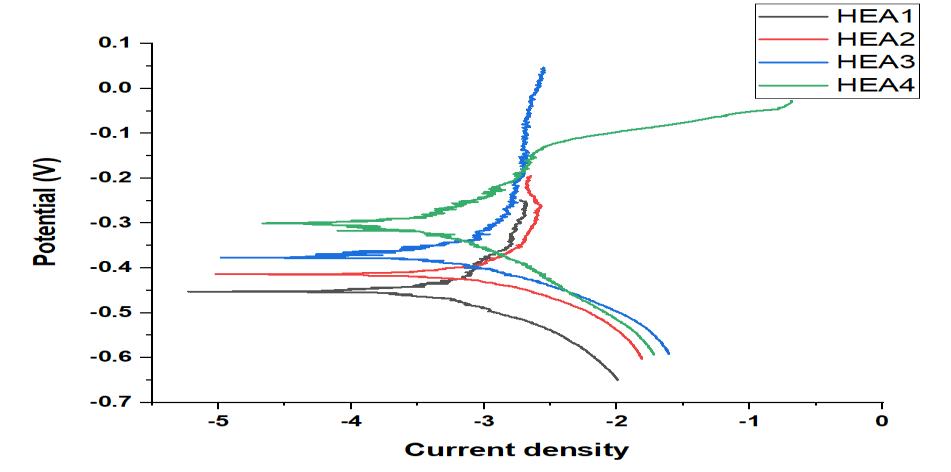
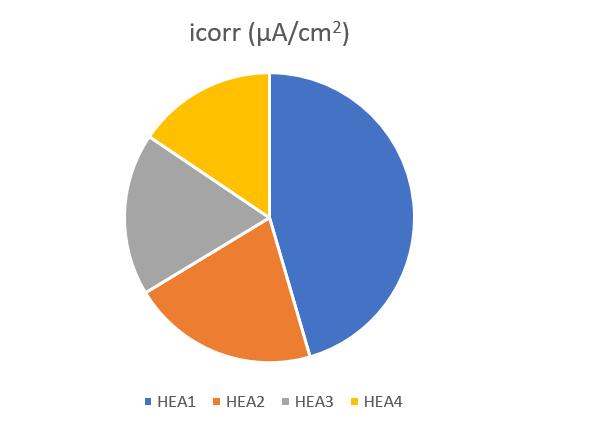
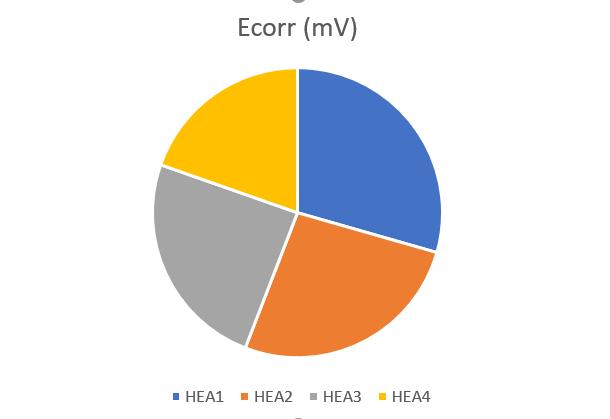
Figure9,Figure10andFigure11 revealthatthecorrosionpotential(Ecorr)increasedandicorrdecreased withtheincrease of Ti content, indicating the positive effect of Ti in controlling the corrosion rate. The increased Ecorr shows the decreased tendencytocorrosion.Theicorrisakinematicfactor,andthedecreaseoficorrvaluemeansthedecreasedthecorrosionrate. For HEA1, the icorr and Ecorr were 2 605.815 µA/cm2 and 452.306 mV, respectively. When increase of Ti in HEA2, Ecorr increased from 452.306 to 406.231 mV and icorr decreased to 1194.371 µA/cm2. With further increase of Ti in HEA4, the corrosionperformancebecamesuperiorastheEcorrvalueincreasedandicorrvaluedecreasedcontinuouslyascomparedto HEA3.TheincreaseinthequantityofTileadtodecreaseinthevalueofcurrentdensity(icorr)indicatesthatthealloysample withhighTicontentformedmorecorrosionresistantfilmonthesurfacein3.5wt.%NaClaqueoussolutionthathinderedthe passageanyionorelectronthroughitselfwhichledtodecreaseincurrentdensity.
International Research Journal of Engineering and Technology (IRJET)

Volume: 09 Issue: 04 | Apr 2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072
Alloy Corrosion rate (mmpy)
HEA1 24.63 HEA2 11.29 HEA3 9.80 HEA4 8.42
The effect of Ti, seen in the present study, can be compared with the previous studies conducted in literature [16, 17]. However, the obtained corrosion parameter values cannot be directly compared because of different environments and different synthesis techniques. For example, Qui et al. [12] revealed that the Ti containing Ti0.3(CoCrFeNi)0.7 HEA showed higherEcorr( 273mV)andlessericorr(0.036μA/cm2)comparingwithTi freeCoCrFeNialloy(Ecorr= 304mV,icorr=0.610 μA/cm2).ItshowsthepositiveeffectofTisimilartothepresentstudy.
AlltheHEAsweresynthesizedsuccessfullyusingmeltingandcastingtechnique AllthesamplesformedsingleFCCphasewith significant peaksof(111),(200),and (220).ThehardnessofHEAsincreased from HEA1toHEA4dueto large Tiatomic size. Thecorrosionresistancein3.5wt.%NaClsolutionenhanced fromHEA1toHEA4duetoincreaseofTicontentwhichhelped inpassivatingthesurfaceeffectivelyandeasily.
Conflict of Interest:AuthorsdeclarenoconflictofInterest
1. B. R. Anne, S. Shaik, M. Tanaka, and A. Basu, “A crucial review on recent updates of oxidation behavior in high entropy alloys,”SNAppl.Sci.,vol.3,no.3,pp.1 23,2021,doi:10.1007/s42452 021 04374 1.
2.B.Cantor,I.T.H.Chang,P.Knight,andA.J.B.Vincent,“Microstructuraldevelopmentinequiatomicmulticomponentalloys,” Mater.Sci.Eng.A,vol.375 377,pp.213 218,2004,doi:10.1016/j.msea.2003.10.257.
3. J. W. Yeh et al., “Nanostructured high entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes,”Adv.Eng.Mater.,vol.6,no.5,pp.299 303,2004,doi:10.1002/adem.200300567
4. M.J. Yao, K.G. Pradeep, C.C. Tasan, D. Raabe, A novel, single phase, nonequiatomic FeMnNiCoCr high entropy alloy with exceptionalphasestabilityandtensileductility,Scr.Mater.72 73(2014)5 8.
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified
International Research Journal of Engineering and Technology (IRJET) e ISSN: 2395 0056 Volume: 09 Issue: 04 | Apr 2022 www.irjet.net p ISSN: 2395 0072

5. Z.Li,K.G.Pradeep,Y.Deng,D.Raabe,C.C.Tasan,Metastablehigh entropydualphasealloysovercomethestrength ductility trade off,Nature534(2016)227 230.
6.T.A.Listyawan,H.Lee,N.Park,Anewguideforimprovingmechanicalpropertiesofnon equiatomicFeCoCrMnNimedium andhigh entropyalloyswithultrasonicnanocrystalsurfacemodificationprocess,J.Mater.Sci.Technol.59(2020)37 43.
7.J.Su,D.Raabe,Z.Li,HierarchicalmicrostructuredesigntotunethemechanicalbehaviorofaninterstitialTRIP TWIPhigh entropyalloy,ActaMater.163(2019)40 54.
8. C. Dai,H. Luo, J. Li,C. Du,Z. Liu,J. Yao, X ray photoelectron spectroscopy and electrochemical investigation of the passive behaviorofhigh entropyFeCoCrNiMoxalloysinsulfuricacid,Appl.Surf.Sci.499(2020)143903.
9. Y.Y.Chen,T.Duval,U.D.Hung,J.W.Yeh,H.C.Shih,Microstructureandelectrochemicalpropertiesofhighentropyalloys a comparisonwithtype 304stainlesssteel,Corros.Sci.47(2005)2257 2279.
10. C.C.Yen,H.N.Lu,M.H.Tsai,B.W.Wu,Y.C.Lo,C.C.Wang,S.Y.Chang,S.K.Yen, Corrosionmechanismofannealedequiatomic AlCoCrFeNitri phasehigh entropyalloyin0.5MH2SO4aeratedaqueoussolution,Corros.Sci.157(2019)462 471.
11. C. Dai, T. Zhao, C. Du, Z. Liu, D. Zhang, Effect of molybdenum content on the microstructure and corrosion behavior of FeCoCrNiMoxhigh entropyalloys,J.Mater.Sci.Technol.46(2020)64 73.
12. Y.J. Hsu, W.C. Chiang, J.K. Wu, Corrosion behavior of FeCoNiCrCux high entropy alloys in 3.5% sodium chloride solution, Mater.Chem.Phys.92(2005)112 117.
13. Y.Z. Shi, B. Yang, X. Xie, J. Brechtl, K.A. Dahmen, P.K. Liaw, Corrosion of AlxCoCrFeNi high entropy alloys: Al content and potentialscan ratedependentpittingbehavior,Corros.Sci.119(2017)33 45.
14. K.H.Lo,C.H.Shek,J.K.L.Lai,Recentdevelopmentsinstainlesssteels,Mat.Sci.Eng.R65(2009)39 104
15. A. Erdogan, K.M. Döleker, S. Zeytin, Effect of laser re melting on electric current assistive sintered CoCrFeNiAlxTiy high entropy alloys: Formation, micro hardness and wear behaviors, Surf. Coatings Technol. 399 (2020) 126179. https://doi.org/10.1016/j.surfcoat.2020.126179
16. Y. Qiu, M.A. Gibson, H.L. Fraser, N. Birbilis, M.A. Gibson, H.L. Fraser, N.B. Corrosion, Y. Qiu, M.A. Gibson, H.L. Fraser, N. Birbilis, Corrosion characteristics of high entropy alloys, Mater. Sci. Technol. Vol. 31, pp. 1235 1243, 2016. https://doi.org/10.1179/1743284715Y.0000000026
17.R.K.Mishra,P.P.Sahay,R.R.Shahi,Alloying,magneticandcorrosionbehaviorofAlCrFeMnNiTihighentropyalloy,J.Mater. Sci.vol.54,pp.4433 4443,2019.https://doi.org/10.1007/s10853 018 3153 z.
Impact Factor value: 7.529 |