International Research Journal of Engineering and Technology (IRJET)

Volume:09Issue:04|Apr2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072

International Research Journal of Engineering and Technology (IRJET)

Volume:09Issue:04|Apr2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072
aM.Tech Scholar, Department of Mechanical Engineering, Sri Sai College of Engineering & Technology, Badhani
bAssistant Professor, Department of Mechanical Engineering, Sri Sai College of Engineering & Technology, Badhani
***
Abstract :Thealuminiumelementisconsideredahighlycorrosionresistantelement,duetowhichitisworthytoinvestigate theAl basedalloysagainstdifferentcorrosionmediumssuchasNaCl,H2SO4,HNO3 etc..TheAl basedalloysarealsopreferred duetotheirlightweight.Presently,thealloyswerepreparedusingmeltingandcastingrouteinan inertenvironment.Allthe Al basedalloyswerecharacterizedusingX raydiffraction,differentialscanningcalorimetry(DSC)andopticalmicroscopy.The XRDtestswereconductedto analyzetheir phaseformationandDSC wasused to estimate the melting pointof all thealloys. thesurfacepropertiessuchashardnessvaluesandmicrographswerealsotaken.Thehardnesstestingapparatuswasusedfor determiningthehardnessvalue,afterwhichthegraphwasplottedtoobservetheeffectofcomposition.Finally,thecorrosion resistanceofalloftheproducedalloysin3.5weightpercentNaClsolution wasanalyzed.Itwasalsopossibletocomparethe corrosion parameters of all of the different alloys. The corrosion current density (icorr), corrosion potential (Ecorr), and corrosionratearethethreeprimarycorrosionparametersinvestigatedinthiswork.
Keywords: Brazingfiller,X raydiffraction,Opticalmicroscopy,Differentialscanningcalorimetry,Corrosion
Manufacturers of thermoelectric devices state that brazing is the most often used technique of attaching components in thermoelectricdevices.Thisflexiblemetaljoiningprocessconnectstwo(ormore)components togetherbyemployingafiller metal as an adhesive and connecting them with a brazing rod (an alloy with a lower liquidus temperature than either component it is used to join). Brazing is a process in which an assembly of components and filler metals is heated to a temperature above the liquidus temperature of the filler metal (for brazing, a minimum liquidus temperature of 450°C is required in order to distinguish the process from soldering, which uses fillers with a liquidus temperature below 450°C), at whichpointthefillermetalmeltsandturnsmolten[1 4]
Recently,variousresearchinitiativessettheobjectivetoprevent,respectivelylimitintermetallicphaseformationbyutilizing high entropy filler materials [5,6]. In earlier investigations, high entropy filler metals were employed in order to effectively combinenickel basedsuperalloysaswellasSOFCs[7,8].Basedonthehighentropynotion,metallicmulti componentalloys with an equal distribution of their alloy components are able to minimize the number of potential occurring phases by generatingarandomsolidsolutionstructure[9,10].AccordingtoYehetal.,ahighentropyvalueencouragesthecreationofa randomsolidsolution(RSS),inwhichthealloycomponentsarerandomlydispersedacrossitslattice.
Specifically, the equimolar high entropy alloy Al Si Sn Zn Cu was investigated as a filler material for the vacuum brazing procedure used to link the Al based super alloy. It is worth noting that the liquidus temperature of Al Si Sn Zn Cu is 1346 degreesCelsius,whichismuchhigherthanthesolutioningtemperatureofMar M247,whichvariesbetween1080and1170 degreesCelsius[11].IthasbeenchosentoalloytheAl 12Sialloyinordertolowerthemeltingpointofthebrazingfiller,which willbevalidatedusingDSCdata.Followingthat,anelectrochemicalpotentiostatwasusedtoexaminethecorrosionresistance ofalloftheproducedalloysin3.5weightpercentNaCl.Itwasalsopossibletocomparethecorrosionparametersofallofthe differentalloys.
value:
Ravijot Singha , Saurabh Sharmab, Sangamdeep Singhb
International Research Journal of Engineering and Technology (IRJET)

Volume:09Issue:04|Apr2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072
Material and Methods:
2.1. Materials: Thephysical characteristicsof the as receivedpowders ofaluminum,copper, cobalt, molybdenum,silicon, tin, copper,andzincemployedinthisinvestigationwereusedtosynthesizethesamples.Theas receivedAl,Co,Mo,Si,Sn,Zn,and Cupowderswereweighedaccordingtotherequiredcompositions(Table1).
Table1Compositionofalloys(wt.%)
Compositions
Cu Al
Balance
2 Balance
4 Balance
Balance
Balance
Firstandforemost,thepowdersweretakenandcombinedintothemortarinaccordancewiththecompositions.Then,usinga 15mmdiameterdiemountedinuni axialcompactionmachinery,thepowders werecompactedtoproducethefinalproduct. The pellets weremadein thismanner and wereoffive distinctcompositions.Inthenextstep,the pelletswere insertedinto thecoppermold,andtherequisitevacuumlevelwasobtained,afterwhichinertargon gaswaspurgedfromthecoppermold. Finally,thepellets were melted five times witha spark, thestrength of whichmay becontrolled by varyingthe current flow rateinthecircuit.Byimpactingelectronswithalargeamountofkineticenergy,thetemperatureofthematerialwillrisevery quicklyveryquickly.
Extensive phase investigation of aluminum based alloy samples was carried out using XRD, which operates on the basis of Bragg's Law. Equipment using Cu radiation was used to conduct XRD experiments on a variety of various compositions. The analysiswascarriedoutinthe2 thetarangeof20o 80o AllXRDpeakswerefittedusingpeakfittingtogetthepeaklocations and full width at half maxima, which were then computed. Differential Scanning is a kind of scanning in which two or more images are compared side by side. For thermal examination of materials in which phase transitions such as melting, glass transitions,orexothermicdecompositionsareinvestigated,thecalorimetrymethodisutilized.Boththereferencesampleand

© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal
Page3658
International Research Journal of Engineering and Technology (IRJET)

Volume:09Issue:04|Apr2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072
the sample on which thermal analysis is to be performed are necessary for this experiment to be completed. The reference sampleshouldhaveawell definedheatcapacityacrossthetemperaturerangethatwillbescannedinordertobeuseful. For the optical microscopy, the sample were polished with SiC abrasive papers, after which polishing was done using velvet clothhavingaluminasuspensiondilutedwithwateronadoublediskpolishingmachine.Forthehardnessofthesamples, the Vickers hardness tester was used. The 5 hardness values were taken, after which the average was calculated. The corrosion tests,thetestswerecarriedoutthreetimesoneachsamplerepeatedlyinNaCl.TheexperimentbeganwiththeplotoftheOCP (Open circuit potential), and after a stable condition had been attained, the polarization curves were constructed using the datafromtheplot.ThevaluesofEcorr andicorr werefoundafterfittingthecurves
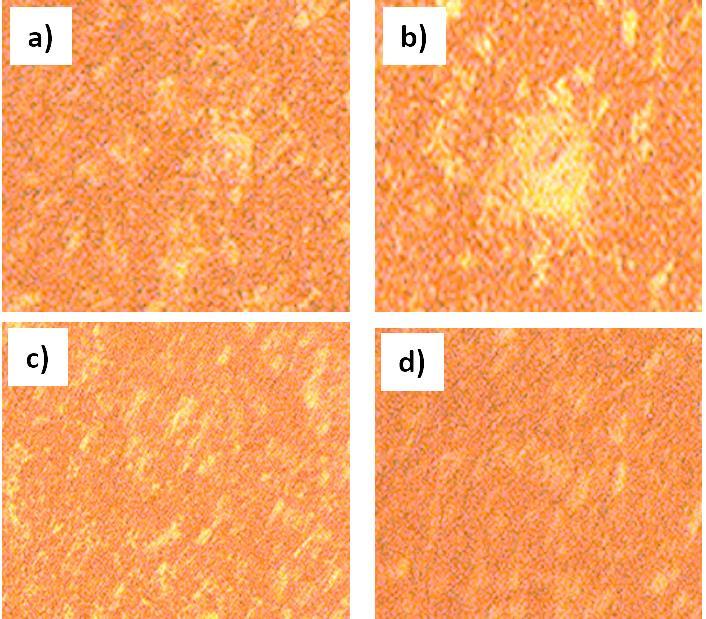
We performed XRD analysis on each and every one of the aluminum based alloys and tested in order to determine which phaseswerepresentinthealloys.Figure2showscrystallinepeaksoftwoseparatephasesofaluminumandsiliconthatmay be distinguished. According to certain theories, the strength of the Si peak has been weakening as a consequence of the creationofsupersaturatedSisolutionsintheAl,aswellastherapidcoolingratesthatwereseenthroughoutthemeltingand casting processes. Because the alloying elements Co, Mo, Sn, Zn, and Cu were completely dissolved and a solid solution produced,itisnotfeasibletoobservepeaksfortheseelementsintheresultinganalysisinfigure2ofC1.Whencomparingthe other alloying elements, including alloys, to the single Si containing alloy, the intensity of the Al and Si peaks in the other alloyingelements,includingalloys,issubstantiallydecreasedincomparisontothesingleSi containingalloy(C0).
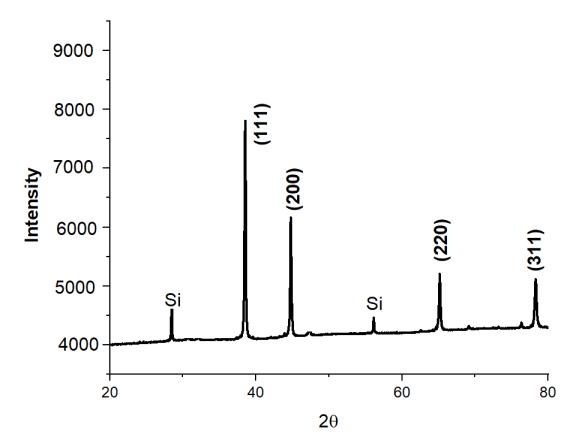
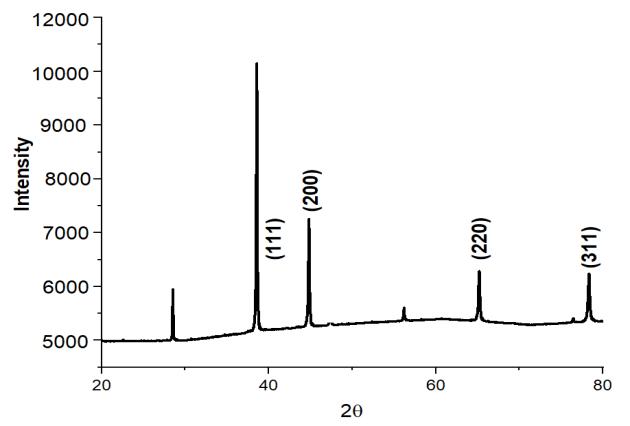
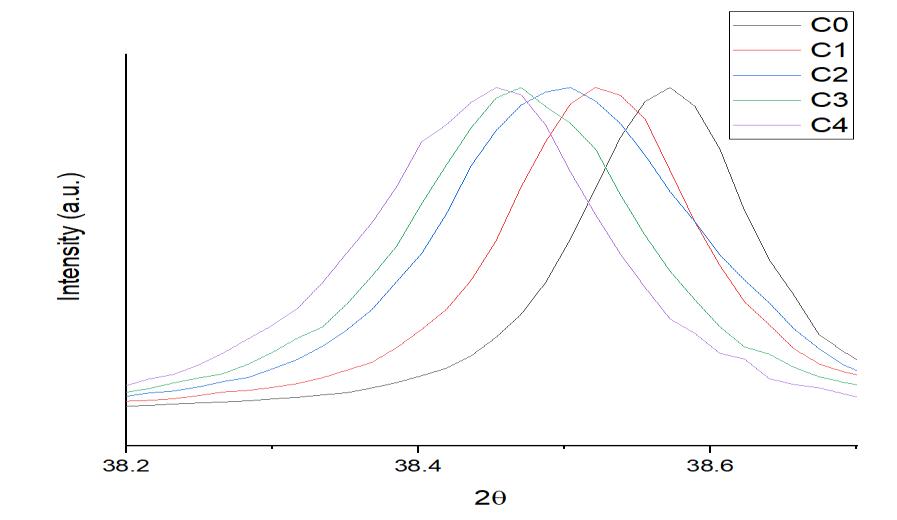
This migration of the most prominent XRD peak of alloys from its original location to its left side indicates that the lattice expansionofAl atomshashappenedasa consequenceofsolidsolutionformationwithalloyingelements(Co,Mo,Cu,Sn, Zn, Si).AstheconcentrationofCuinthesolutionrose,thelatticestructurecontinuedtogrowasseeninfigure4.
value:
International Research Journal of Engineering and Technology (IRJET)

Volume:09Issue:04|Apr2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072
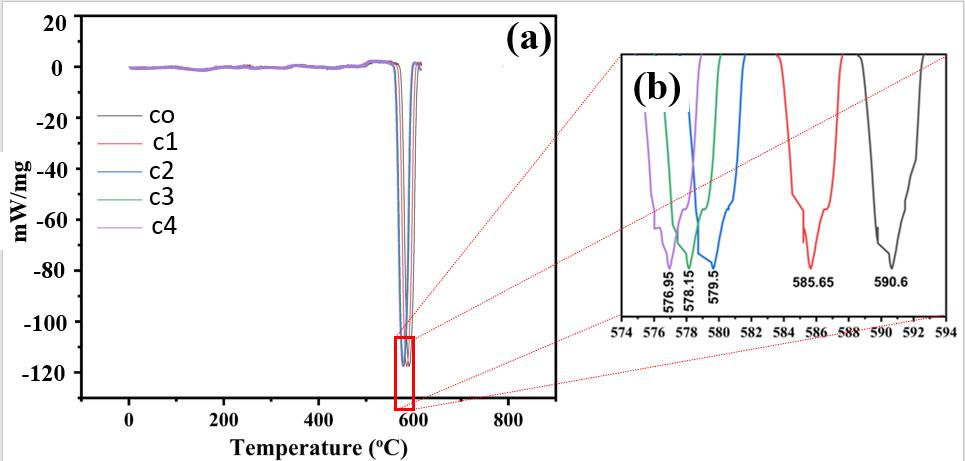
TheDSCstudywascarriedoutonallofthe Al basedalloysthatarepresentlybeingsynthesized.Allalloysweresubmittedto DSCtestingupto700degreesCelsius.Accordingtoresearch,590.6degreesCelsiuswasrevealedtobethemeltingpointofC0 alloy.Themeltingpointofthealloysdecreasedwhenalloyingwasintroduced.Furthermore,whentheCuconcentrationgrew, the melting point decreased. Because filler materials should have the lowest melting point possible, it means that the Cu additionmakesthealloymoresuitableforuseasafiller.TheopticalmicrographsofallthealloysareshowninFigure6.
International Research Journal of Engineering and Technology (IRJET)

Volume:09Issue:04|Apr2022
e ISSN: 2395 0056
p ISSN: 2395 0072
3.4. Electrochemical analysis of Al based alloys in 3.5% NaCl solution
Afterthepolishedsamplesofaluminum basedalloyswerelinkedwithcopperwireandsubsequentlymountedinepoxyresin, just one surface with a surface area of one centimeter square (cm2) was subjected to corrosive conditions. The sample was usedastheworkingelectrode,anditwasplacedinathree electrodeglasscellwithacounterelectrode(platinummesh)anda referenceelectrodetoconducttheexperiment(SCE).Eachexperimentstartedwithanhourofocp(opencircuitpotential)to allowthesampletostabilize.Thepotentiodynamicpolarizatoncurvewasthengeneratedbyscanningtheelectrodesatarate of 0.5 mV/s. Using the tafel fit, it was possible to determine the electrochemical parameters of the sample, such as the corrosionpotential(Ecorr)andthecorrosioncurrentdensity(icorr).Becausethecorrosionrate(CR)iscloselyrelatedto the corrosioncurrentdensity(icorr),thevalueoficorr wasusedtocomputethecorrosionrate(CR).InordertocomputeCR,itwas required to take into account two more factors: density and equivalent weight. Figure 7 shows the potentiodynamic polarization curves of all of the alloys, which were created in a 3.5 weight percent NaCl solution in order to highlight the influenceofCuoncorrosionbehavior.Thedropincorrosionpotentialimpliesadecreaseinthesusceptibilitytocorrode,and the decrease in current density indicates a decrease in the rate of corrosion. The electrochemical corrosion parameters are listedinTable2.
International Research Journal of Engineering and Technology (IRJET)

Volume:09Issue:04|Apr2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072
Figure7.Potentiodynamicpolarizationcurvesofsamplesin3.5wt%NaClaqueoussolution
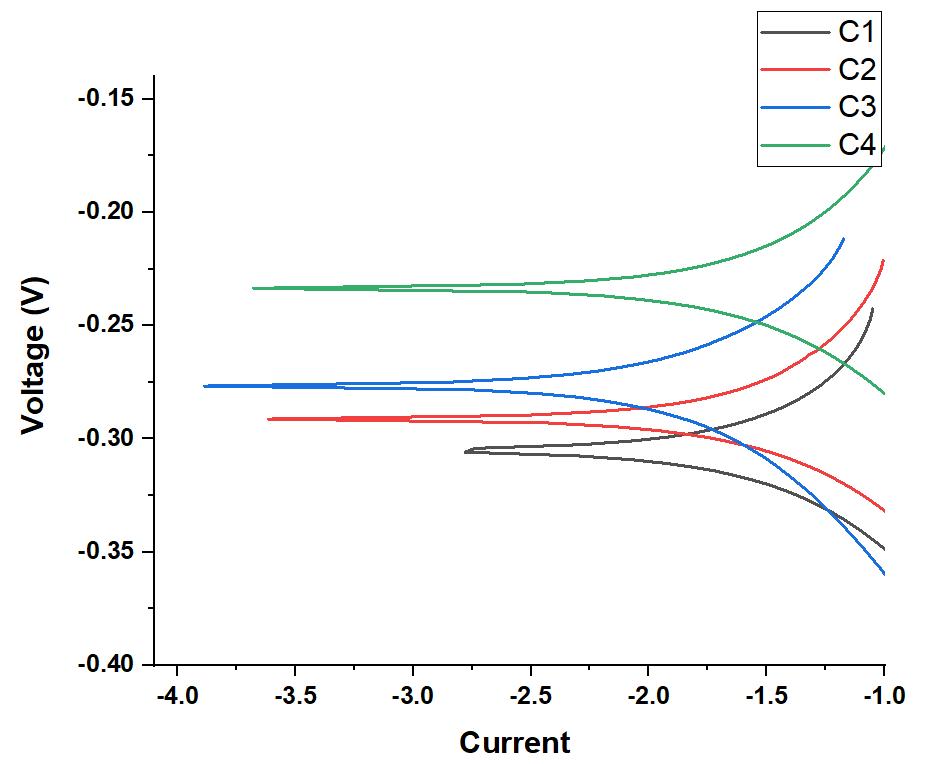
Table 2 Potentiodynamic polarization results of samples in 3.5 wt % NaCl aqueous solutions
Alloy Ecorr (mV) icorr (µA/cm2)
Corrosion rate (mmpy)
C1 305.66 38676.80 365.7055 C2 290.33 36575.32 345.8351 C3 270.80 22330.34 211.1428 C4 234.85 47467.45 448.8248
Conclusion: TheAl basedalloysweresuccessfullysynthesizedusingmeltingandcastingtechnique. ThemeltingpointofAl based alloy decreased with the addition of alloying elements and further with the increase of Cu content. But the significant meltingtemperaturedropwasthereuptocertainincreaseofCu%.ThedensityofalloysincreasedwithincreaseofCucontent. Inaddition,theactualdensitywasslightlylessercomparingwiththetheoreticaldensityduetopresenceofsomeporosity.The hardness of Al based alloys increased with the increase of Cu wt %. The corrosion performance was superior in C3 alloy in 3.5%NaClthanotherinvestigatedalloysinthisstudy.
Conflict of Interest: Authorsdeclarenoconflictofinterest.
2022, IRJET | Impact Factor value: 7.529
ISO 9001:2008
International Research Journal of Engineering and Technology (IRJET)

Volume:09Issue:04|Apr2022 www.irjet.net
e ISSN: 2395 0056
p ISSN: 2395 0072
1. T. Onzawa, A. Suzumura, and M. Ko, “Brazing of titanium using low melting point Ti based filler metals,” Welding Journal,vol.462,1990.
2. A.E.ShapiroandY.A.Flom,“Brazingoftitaniumattemperaturesbelow800°C:reviewandprospectiveapplications,” DVSBerichte,vol.243,p.254,2007.
3. B.S.Murty,J.W.Yeh,.;S.Ranganathan,”High.EntropyAlloys,1sted.”;Butterworth Heinemann:London,UK,2014.
4. M. Way, J. Willingham, R. Goodall, Brazing filler metals, Int. Mater. Rev. 0 (2019) 1 29. https://doi.org/10.1080/09506608.2019.1613311
5. Tillmann W, Wojarski L, Manka M et al. (2018) Eutectic high entropy alloys a novel class of materials for brazing applications, Proceedings from the International Brazing & Soldering Conference, 15th to 18th April 2018, New Orleans,pp142 148
6. Hardwick L, Rodgers P, Pickering EJ et al. (2019) Development of novel nickel based brazing alloys, utilising alternative melting point depressants and high entropy alloy concepts, Proceedings from Brazing, high temperature brazinganddiffusionbonding,12thInternationalConference,21stto23rd May2019,Aachen,pp7 17
7. TillmannW,WojarskiL,UlitzkaTetal.(2019)Brazing ofhightemperature materialsusingmeltingrangeoptimized filler metals based, Proceedings from Brazing, high temperature brazing and diffusion bonding, 12th International Conference,21stto23rdMay2019,Aachen,pp1 6
8. CantorB,ChangITH,KnightPetal(2004)Microstructuraldevelopment inequiatomicmulticomponentalloys.Mater SciEngA375 377:213 218.https://doi.org/10.1016/j.msea.2003.10.257
9. ZhangLX,ShiJM,LiHWetal(2016)InterfacialmicrostructureandmechanicalpropertiesofZrB2SiCCceramicand GH99 superalloy joints brazed with a Ti modified FeCoNiCrCu high entropy alloy. Mater Des 97:230 238. https://doi.org/10.1016/j.matdes.2016.02.055
10. Yeh J W (2013) Alloy design strategies and future trends in highentropy alloys. JOM 65(12):1759 1771. https://doi.org/10.1007/s11837 013 0761 6
11. Baldan R, da Rocha RLP, Tomasiello RB et al (2013) Solutioning and aging of MAR M247 nickel based superalloy. J MaterEngPerform22(9):2574 2579.
Impact Factor value: 7.529