International Research Journal of Engineering and Technology
(IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072

(IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
Mr. Yash Shah1 , Mrs. Raksha K. Patel2 , Dr. Anand Mankodia3 , Dr. Vipul A. Shah4 , Dr. ManishThakker 5
1Research Scholar, M. Tech Biomedical Engineering, U V Patel College of Engineering, Ganpat University, Kherva, Gujarat, India.
2 Associate Professor, Department of Biomedical Engineering, U V Patel College of Engineering, Ganpat University, Kherva, Gujarat, India.
3Associate Professor, Department of Electronics & Communication Engineering, U V Patel College of Engineering, Ganpat University, Gujarat, India.
4 Professor, Department of Instrumentation & Control Engineering, Dharmsinh Desai University, Nadiad, Gujarat, India.
5 Professor, Department of Instrumentation & Control Engineering, L. D. College of Engineering, Ahmedabad, Gujarat, India. ***
Abstract - Most common site for cancer is prostate, for example, of the 184,500 newly diagnosed instances of cancer in men which constitutes 29% of new cases. The patient's age, general health, the type and stage of cancer present, the treatment's potential side effects, and the patient's and doctor's personal preferences are all important considerations when deciding on a course of action. In this article, we will take a look back at the main imaging techniques used to diagnose and localize prostate cancer, including multiparametric ultrasound (US), multiparametric magnetic resonance imaging (MRI), MRI-US fusionimaging, and positron emission tomography (PET) imaging. The biological and functional properties oftumors thatjustify theapplicationof a given imaging modality are given primary consideration. T2-weighted MRI and anatomical grayscale US can reveal alterations in tissue architecture. Doppler and contrast-enhanced ultrasound (US) as well as dynamic contrast-enhanced magnetic resonance imaging (MRI) take advantage of the fact that tumor growth is aided by angiogenesis. Clinical staging with the DRE andPSA isnot very precise; however, imaging modalities like TRUS and MRI can improve this.
Key Words: Prostate, Cancer, Treatment, Fusion, Imaging.
In men, prostate cancer is the second most prevalent type of cancer that ultimately results in death. The rate of prostate cancer, which is measured in instances per 100,000 males, has remained reasonably stable at 165. Early diagnosis and treatment are credited for the 31% decline in the age-adjusted death rate that has occurred since 1990 [1].Thisdecline canbeattributabletothefact thatmorepeoplearelivinglonger.Thediagnosisofprostate cancer has been changed to a lower grade, organ-confined disease as a result of prostate screening with digital rectalexamination(DRE)andprostatespecificantigen(PSA)[2,3].Thishascontributedtotheoverdetectionand overtreatmentofprostatecancerbyatleast30 percent[4].RecentresearchconductedbyEtzioniandcolleaguesestimatedthat10%ofmenwithlow-gradeprostatecancer underwentunnecessaryradicalsurgery,while45%ofthesesamemenunderwentunnecessaryradiationtherapy.Asaresult of the publication of the 10-year results of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial demonstrating that there was no reduction in mortality with screening, the new challenge is in differentiating clinically relevanttumorsfromonesthatmayotherwiseneverhavebecomeevidentifitwerenotforscreening.Becauseoftherapid advancement of imagingtechnologies,prostatecancermaynowbedetectedandstagedmoreaccurately,whichpavesthe wayfor moretargetedtreatmentandsubsequentmonitoring.However,thecorrectuseofimagingisdifficulttopindown due to the numerous contentious studies that can be found in the published literatureconcerning each of the modalities and the utilities that they offer. In this section, we will cover the imaging techniques that are considered to be more established,andwewillreviewvariousimagingtechniquesthatshowpromiseforthefuture.
(IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
Inordertosetthestageforourdiscussionofthevariousimagingmodalitiesfortheprostate,wewillfirstbrieflydigressto coverthemorphologyoftheprostate,pathologygrading,andtherapychoicesforPCa.
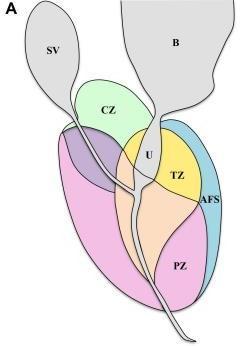
The structure of the prostate. Therectumcoverstheprostateglandfrombehind,whilethebladdercoversitfromabove.The urethraispartiallyencircledbythegland.Basereferstotheendoftheglandclosesttothebladder,whereasapexdescribes theend oftheglandclosesttotheexternalurethralsphincter.Theprostateisdividedinto four anatomical regions called McNeal zones: the peripheral zone (PZ), which contains about 70% of glandular tissue;thetransition zone(TZ),which containsabout5%ofglandulartissue;thecentralzone(CZ),whichcontainsabout25%ofglandulartissue;andtheanterior fibromuscularstroma,whichcontainsabout0%glandulartissue(Fig.1A).PCaincidenceratesareasfollows:68%inthePZ, 24%intheTZ,and8%intheAZ(CZ).4Image-guidedinterventionalprocedures,suchasaprostatebiopsy,makeitsimple toidentifytheseareas.
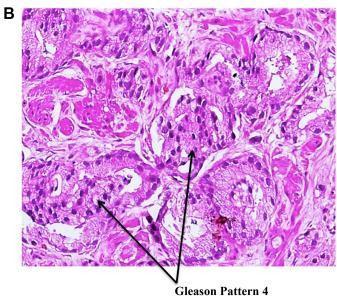
Grading of PCa pathology. Aswaspreviouslynoted,aprostatecancerdiagnosismayonlybemadewithabsolutecertainty withanimage-guidedprostatebiopsy.Thebiopsysamplestakenduringtheprocessaregradedforcelldifferentiationand cancerous aggressiveness using the primary, secondary, and total Gleason scores (Fig. 1B). Patients may be defined as having clinically significant malignancy if their specimens have a higher histopathological Gleason score (usually total Gleasonscore7orprimaryGleasonscore4).Ontheotherhand,aclinicallyinconsequentialtumorisonethatislocalizedto one organ, has a low-grade histology (a total Gleason score of 6 with no Gleason component 4), and has a gross tumor volumeof0.5cc.[5]
Alternatives for treating prostate cancer. ThosewhohavebeendiagnosedwithclinicallysignificantPCamaychooseto treat it with radical prostatectomy (RP), whole-gland radiation therapy (RT), or hormonal therapy. Urinaryincontinence and impotence are two undesirable side effects that somepatients withterminal illnesses may experience as a result of therapeutic therapy. Serum PSA levels, imaging, and rebiopsy can be used together to detect disease recurrence, if any, after treatment. Active surveillance or watchful waiting programmes are commonly used to track low-risk or clinically inconsequentialdiseases.Theseprogrammesmayinvolveimagingtotrackdiseaseprogressionand/orserialbiopsiestolook forchangesovertime.TreatmentoptionsforlocalizedPCa areconstantlyevolving,andrecentresearchsuggeststhatimageguidedfocusedtherapyormalelumpectomymaybecomeincreasinglycommoninthenearfuture
Figure -1: (A) Prostate zonal anatomy is depicted with the McNeal zones in sagittal view. SV: seminal vesicle (gray), B: bladder (gray), CZ: central zone (green), U: urethra (gray), TZ: transition zone (yellow), AFS: anterior fibromuscular stroma (blue), and PZ: peripheral zone (pink) (B) Immunostainedbiopsy specimen demonstrating Gleason pattern 4 adenocarcinoma of the prostate.

e-ISSN:2395-0056
Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
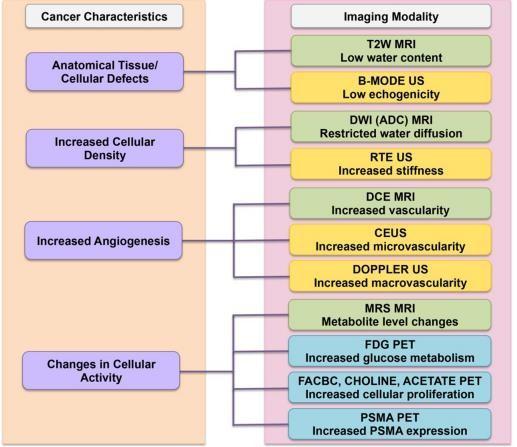
ForthedetectionandlocalizationofPCa,imaginghasrecentlybecomethestandard.US-basedimaging,mpMRI,mpMRI-US fusion imaging, and PET imaging are the current leading modalities for image-guided diagnosis of PCa. The underlying tumor's biological behavior will determine the imaging mode of choice. As shown in Figure 2, we summarize how the characteristicsofthetumorobservedcorrelatetotheimagingmodalitiesused.Inthepartsthatfollow,we'llalsodivedeeper into each of the specific correspondences. Table 1 summarizes the main imaging modalities, including their primary therapeuticapplicationsaswellastheirprosandlimitations.
WhenitcomestoimagingPCa,anatomicalultrasoundimaginghasbeenaroundthelongestandisstillthegold standard. FunctionalimagingtechniquesdevelopedintheUnitedStateshaverecentlypiquedthecuriosityofthescientificcommunity. WeexaminethemanyUSimagingapproachesthatarecurrentlyleadingtothecreationofanmpUS-based approachforPCa detectioninthesectionsthatfollow.
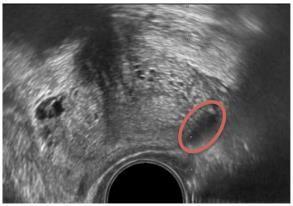
B-mode or grayscale U.S. The most common imaging method for detecting PCa is grayscale or standard B-mode US. BmodeUSisusefulfordistinguishingthezonalarchitectureoftheprostate,withtheoutsidePZappearing moreechogenic thantheinteriorCZandTZ.Grayscaletransrectalultrasonography(TRUS)imagingwasfirstusedbyHodgeetal.toguidesix biopsyneedlesintotheprostatein1989.Sincethen,randomandsystematicprostatebiopsieshavebeenthegoldstandard for detecting PCa,and this method has served asitsfoundation,alongwiththe additional targeting of hypoechoic lesions (cancertissuewithcellulararchitecturedefectsappearslessechogenicthannormaltissueongrayscaleUS,asshowninFig. 3). [6,7] An extended sextant 12-core biopsy, aiming for the apical and lateral parts of the PZ where the cancer is most likelytobehiding,hasbeenthesubjectofnumerousdiscussionsonhowtomaximizeitslikelihoodofdetectingcancer.[8]
The use of traditional B-mode anatomic US imaging for PCa has various drawbacks. Prostatitis, inflammation, and benign prostatichyperplasia(BPH)canallmaketheprostateappearhypoechoiconUSimaging,justlikecancerouscells.Inaddition, early-stagecarcinomas can have an isoechoic appearance as compared to later stages because of the greater amount of normal glandular tissue present at that time. Up to 60% of morphologically worrisome lesions inthe UnitedStates are knowntobebenign,while21%-47% of tumors may be missed on aninitialbiopsy. [9-10] Since random and systematic samplingschemesarenottumororpatientspecific,theycan resultintheunintentionaldetectionofclinicallyinsignificant PCa(low-volumecancerwithatotalGleasonscore7)[11-12]and theundergrading of thedisease's aggressiveness dueto insufficientsamplingofthebulkofthetumor.[13]
Figure 2. Correspondence of imaging modality to cancer characteristics. The left column shows thebiological cancer characteristics and their correspondence to the choice of imaging modalities

e-ISSN:2395-0056
Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
Table 1. A summary of clinical usage, advantages, and disadvantages across imaging modalities for PCaimaging.
ultrasound-based initialdetectionand diagnosis Office-based,widely available,inexpensive, real-timeimaging
excellenttissuecontrast for identificationof
limitedtissuecontrast betweencancerousand benigntissue
mpuS-basedapproach (rte,ceuS)mayimprove contrast mpmri-based initialdiagnosisand recurrence, active
expensiveduetoin-bore time,lackofreal-time imaging,requires advanced
alternativein-bore optionswithrealtimeimagingbeing
clinicallysignificantPCa training developed mpmri-ultrasound fusion-based initialdetectionand diagnosis,active surveillance
surveillance,staging, metastatic involvement
Office-based,com-bines multimodality information
relativelycostly,requires eitherfusion-devicespecifictrainingorample experiencetoperform cognitivefusion, registrationerrorsduring mri-ultrasoundfusion
gainingpopularity globally,butfurther improvementsto minimizeregistration errorsneeded pet-based Staging,recurrence, metastaticspread offersancillary informationfortumor staging,characterization andmetastatic involvement
expensive,technological (e.g.attenuation correction)and/orclinical challenges(e.g.radiation exposure)
developmentofspe-cific radionuclidesisan ongoingendeavor
US picture analysis with the help of a computer. AlterationstocellstructureareahallmarkofPCa,andthesealterations influence the way US signals backscatter. This information can be utilized to distinguish between cancerousandnormal tissue.Severalproductsonthemarketperformreal-time,computer-basedanalysisoftheseUSsignals;forinstance,onecan usestatisticalanalysisofrawUSdatatocreateaprostatehistogram(ProstateHistoScanning[PHS]),whileanothercanuse anartificialneuralnetworktoanalyzedigitalB-modeultrasoundimages(ANNA/computerizedtransrectalultrasound[CTRUS]).
Fig – 3 B-mode US image of the prostate depicting hypoechoic lesion (red oval).

e-ISSN:2395-0056
Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
Prostate HistoScanning. PHSisamethodforidentifyingpotentiallymalignantprostatelesionsthroughstatisticalanalysisof rawbackscatteredUS.EarlyresearchbyBraeckmanetal.on29patientsundergoingRPdemonstratedasensitivityof100%, specificityof82%,NPVof80%,andPPVof100%forlesionsmorethan0.5mLinvolume.[14] Twenty-sevenpatientswere analyzedinastudybySimmonsetal.,whofoundthatPHShadasensitivityof90%andaspecificityof72%forlesionsatleast 0.2mLinsize.PHSfunctionedpoorlyinthefrontprostateandoptimallyinthecentralandposteriorhalvesofthegland.[15] Thedetectionoflesions0.1cm3in98menwasshowntohaveasensitivityof60%andaspecificityof66%byMaceketal.17 Despite Javed et al best .'s efforts, PHS has been shown to be unreliable in typical clinical settings across three separate investigationsinvolvingatotalof105men.Intheseinvestigations,PHSwasusedtoevaluatepatientsbeforetheyhadRP(n= 24),TTB(n=57),oratransperinealtemplatebiopsy(TGB;n=24).CancerwasfoundatahigherrateinPHS(38.1%)than inTGB(62.5%)andTTB(13.4%vs.54.4%).Furthermore,PHSandRPpathologyyieldedinconsistentresultswhencomparing tumorvolume estimates. For 148 patients, Schiffman et al. also discovered no connection between RP and PHS tumor volumes.However,atpresent,thereliabilityofthismethodforPCadetectioniscompromisedbyalackofstrong,consistent clinicaldata.[16]
The use of transrectal ultrasound imaging and artificial neural networks. Classifyingtissueareasasmalignantorbenign usingANNAofC-TRUSimagesisattheheartoftheANNA/C-TRUSsystemforPCadetection.Thedoctorperformsastandard B-mode transrectal US exam of the prostate, sends the digital images to a central server, and receives them back with potentially cancerous spots highlighted. The user is then able to direct biopsies to the selected areas. To train the ANNA classifier,weusedsetsofcorrelatedUSimagesfromRPspecimensannotatedwithpathologicalinformation.Halfofthe132 menstudiedwhohadonetosevennegativeconventionalgrayscaleUSbiopsieswerefoundtohavecancerbytheANNA/CTRUS method. Conventional US-guided systematic approacheshaveahistoricaldetectionrateof7%forPCainmaleswith repeatbiopsy.[17]Anotherresearchof75 biopsy-naivemenfoundthat41%ofthemhadPCa,whichwasdetectedbythe ANNA/C-TRUS.Strunketal.showed that using mpMRI in conjunction with C-TRUS enhanced the identification of PCa in high-riskindividuals.Largermulti-centertrialsarerequiredtogaugethetruetherapeuticefficacyofANNA/C-TRUS,which hasshownencouraginginitialresults.
Doppler US. Over-vascularizationandnewbloodvesselgrowtharecommonfeaturesofPCa.WhenUSwavesfromatransducer hitthemovingredbloodcellsinthebloodvessels,thereflectedwavesalterinfrequencyinamannerthatisproportionateto thecells'velocities,asdemonstratedbytheDopplereffect.Changesinfrequencyarecolor-overlayedonreal-timeB-modeUS imagestoshowareasofenhancedperfusion,suchasthoseseenintumors.The coreofPCa detectionusingcolorDoppler ultrasound(CDU)imagingisfocusingontheseareasof enhanced blood flow.Anothervariation,knownaspowerDoppler ultrasonography(PDU),visualizesthetotalintegratedDopplerpowerincolor.PDUimagingismoresensitivetoperfusion thanCDU,andhencemaybebetterabletodetect reducedbloodflowinsmallerdiameterbloodarteries,butatthecostof losing the sensation of flow direction. Whenitcomes todetectingPCa, Dopplerimaginghas mixedresults.Therewas no advantagetoemployingDopplerimagingforPCadetectioninastudyof62patientsthatcomparedhigh-frequencyCDUand PDUtorandomsextantbiopsy.[18]Okiharaetal.usedPDUimagingtoexamine107maleswithelevatedserumPSAlevels. The ultimate sensitivity, specificity, PPV, and NPV for PDU imaging indicating a lesion were 98%, 78%, 59%, and 99%, respectively.Inastudyincluding243males,Sauvainetal.demonstratedthatPDUimaginghasasensitivityandspecificity of 45% and 74%,respectively, for diagnosing low-risk PCa.[19] Doppler US imaging for PCadetectionis restricted byits inabilitytodetect blood flow in capillaries smaller than0.1 mm9.Neo-microvessels,whichrangein sizefrom10-50m on average, proliferate at an accelerated rate during cancer development. Doppler imaging may only be useful for detecting cancerswithadvancedstagesandhighGleasongradessinceitcanonlydetectincreasedbloodflowinlargermacrovessels.
Ultrasound imaging with contrast medium. Angiogenesisanddisorderedneovascularization,whichcangreatly increase microvasculardensity(MVD),arefrequentlyobservedalongsidetumorgrowthandprogressionwithintheprostate.Contrastenhanced ultrasound (CEUS) imaging aims to detect this improvement in MVD. During a biopsy with CEUS imaging, microbubblesloadedwithahighlyechogenicgasareinjectedintravenously.Thesemicrobubblesarethesamesizeasred blood cells, so they may travel through (and be imaged by) the tiny bloodvessels that supply tumors. CEUS has this advantageoverDopplerUSimaging,whichcanonlyfocusontheflowin bigger macrovessels due to its lower resolution. Asymmetrical fastorfocusedenhancementistypicallyusedto identifymalignancyusingCEUS.CEUSquantitativeanalysis canbeperformedbytrackingtheconcentrationoftheUScontrastagent overtimeorbycomputingtheagent'sdispersion kinetics as it travels through the microvasculature. [20] Li et al. conducted a meta-analysis of 16studies involving 2624 patientsandconcludedthat CEUSimaginghasacombinedsensitivityandspecificityof70%and74%,respectively,forthe identificationofPCa.

e-ISSN:2395-0056
Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
Real-time elastography. It's common knowledge that PCa tissue is more rigid than regular prostate tissue. Increased stiffness of malignant tissue in the prostate is caused by the increased cellularity, increased micro vascularity, loss of glandulararchitecture,reductioninacinararea,andincreasedcollagendepositioninthestromaaroundcancer.[21]Actually, thephysicalDREreliesontheclinicianutilizinghisorherindexfingertopalpatetheposteriorPZoftheprostateinorderto discover firmer or stiffer aberrant masses of cancer tissue. Real-time elastography (RTE) is an improved and more trustworthy whole-gland option for finding these stiffer regions within the prostate gland. Mechanical stimulation of the prostate tissue is induced by the physician, and the resulting response is imaged, typically with real-time US, in RTE imaging. Strain elastography (SE), acoustic radiation force impulse (ARFI) imaging, and shear wave elastography are all examplesoftechniquesthatcanbecategorizedaccordingtotheexcitationmethod(SWE).
Strain elastography. A US probe is used to mechanically compress and release the prostate tissue repeatedly in SE. Differentialstrainordeformationoftheprostatetissueisgenerated,anditisshownasacolormapsuperimposedonrealtimeB-modeUSpictures.Thelivecolormaphelpsdistinguishstiffertissuefromsoftertissueandenablesthecliniciantoguide thebiopsyneedletothestifferareas(andhencemorelikelytobemalignant)intheprostate.Inameta-analysis,Zhangetal. lookedatsevenstudiesthatcomparedtheaccuracyofdiagnosticSEwithRPspecimensasgoldstandards.Overall,thepooled sensitivityandspecificityinthissampleof508maleswas72and76percent,respectively.[22]ComparisonsbetweenSEand MRI-guidedbiopsieshavealsobeenmade.Intheirstudyof33patientswithPZlesions,Aigneretal.foundthatstrainRTE andT2-weightedMRIhadidenticalsensitivityandNPV.Fiftypatientswithbiopsy-provenmalignancywhounderwentstrain RTEandmpMRIexamstoidentifyPCawereevaluatedinaretrospectiveresearchbyPelzeretal.Therewasarelationship between the outcomes and RP samples. Although the authors noted that the MRI findings may have been biased by the prior biopsies that generate hemorrhage abnormalities on MRI, the sensitivity of strain RTE was greater than mpMRI (92%vs.84%).Interestingly,MRIworkedbetterinthebaseandTZ,whilestrainRTEdidbetterinthedorsalandapicalto middleregionsoftheprostate.ByfusingMRIandstrainRTEtopinpointworrisometumors,Brocketal.increasedspecificity in a population of 121 previously negative men receiving fusion biopsies. For MRI/strainRTE fusion, the sensitivity and specificitywere77.8%and77.3%,respectively,comparedto74.1%and62.9%forMRI alone.Free-handcompressionsand decompressionswiththeendorectalUSprobeposeasignificantchallengetoSE becausetheyrelyheavilyon the skill ofthe operator. To counter this, Tsutsumi et al looked explored employing inflated balloons for applying more uniform compressions.ThedifficultyofSEiscompoundedbythefactthatthelivecolormappingsaresubjectivetoeachindividual operator.Thecolormapsareautomaticallyscaledtothemaximumandloweststrainsinaspecific2Dimagingplane,anditis toughtoestablishanabsolutequantitative3D threshold ofstiffnessthatcandiscriminatemalignant frombenigntissuein theentiregland.
Impulse imaging using acoustic radiation force Theprostatetissueisimagedusingultrashort(1millisecond)focusedUS beams of high intensity during ARFI imaging. Tissue is displaced due to an acoustic radiation force generated when momentum is transferred from the acoustic US waves to the propagating medium. Next, the USinquiry measures the resulting shift.It's thesameprincipleas withstrainimaging: tumors and otherstifferpartsof theprostatewillcause less displacementthanhealthytissue.Bydisplayingacolor-codedmapofdisplacementsinreal-time,thedoctorcanbiopsithese areas with pinpoint accuracy. ARFI's key benefit over SE is that it does notrequire skilled manual compressions and decompressions of the prostate. On nine humanprostatespecimens,Zhai et al. [23] used ARFI imaging to differentiate betweenMcNealzones,BPH,calcifications,atrophy,andmalignanttumors.Aninvivostudyinvolving19individualsbefore prostatectomy confirmed these findings. In both trials, however, the restricted depth penetration of the ARFI pulses hamperedthedetectionofanteriorPCa.
Shear wave elastography. InSWE,ashearwaveisproducedintheprostatebymeansofacousticradiationforce, andits velocityissubsequentlymeasured.SincetheYoung'smodulus(ameasureoftissuestiffnessreportedinkPa)isinverselyrelated totheshearwavevelocity,SWEcanbeusedtogenerateaquantitativeimageoftissuestiffness. SWEimaging,incontrastto SE, does not necessitate manually pressing the probe, and it is quantitative as well. Thesetwofeatures makeSWEmore transferableandlessdependent ontheskilloftheuser.A sensitivityof96%, specificity of 96%, PPV of 69%, and NPV of 100% were obtained in an initial SWE evaluation study of 53 males utilizingaYoung's modulus threshold of 37kPato distinguishbenignfrommalignanttissue.Usingathresholdof35 kPa,Correasetal.foundasensitivity,specificity,PPV,and NPVof96%,85%,48%,and99%,respectively,inareal- timeSWEresearchincluding1040PZsextantsin184men.For60 patients,Boehmetal.usedwhole-glandSWEtodetermineathresholdof50kPabeforeperformingaprostatectomy.They found an 81% sensitivity, 69% specificity, 67% PPV, and 82% NPV. [24] In a research of 50 men, Ahmad et al. found a correlationbetweenrisingYoung'smodulusandrisingGleasongrade,withasensitivityandspecificityofaround90%for both.However,unlikepreviousinvestigations,thisonefoundsignificantlydifferentabsolutestiffnesslevelsbetweenbenign andmalignanttissue(75kPavs.134kPa,respectively).FirstresultsfromSWEimaginghavebeenpromisingand multiple

e-ISSN:2395-0056
Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
investigations have indicated that there is a considerable difference between the Young's modulus of benign and canceroustissue.However,ahardnumericalcutoffbetweenthetwohasyettobeestablished.
Multiparametric US. AllUS-basedimagingapproachesleveragetumorbiologypropertiesincludingincreasedvasculature, stiffness,etc.Researchershaverecently usedmpUStoimprovetargetingspecificitybycombiningthese functional methods. Aigner et al. sampled five targeted cores in 133 men using RTE and CEUS. Biparametric detection yielded 59.4% cancer detection. In 150 men, Xie et al. used grayscale, Doppler, and CEUS imaging to identify 49%. mpUS recognized more PCa patients than grayscale, power Doppler, and grayscale plus power Doppler (P = 0.002, 0.001, and 0.031, respectively). Brock et al. evaluated RTE and CEUS pathology in 86 patients with whole-mount sections. RTE and CEUS reduced false positivesfrom35%to10%andboostedPPVfrom65%to 90%.mpUShasshownearlypromise,butmulticentertrialswith biggerpatientcohortsareneededtodevelopit.
MMRI.
MRIforPCadetectionhasusedamultiparametricapproach,unlikeUS-basedmethods.Anatomicalsequences(T2- weighted MRI)arepairedwithatleasttwofunctionalsequences(DWIanddynamiccontrast-enhancedimaging)toimagethetumor's biological features. MR spectroscopy, another functional MRI method, may be included in theacquisition protocol for facilitieswithexpertise.Theseimagingmethods,calledmpMRI,providesimultaneoustumor
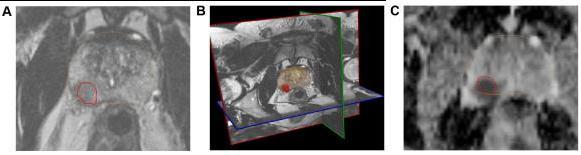
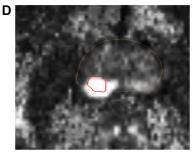
T2-weighted imaging assessment. High-resolution axial, sagittal, and coronal T2-weighted imaging (T2WI) sequences showprostatezonalanatomyandsofttissuecontrast(Fig.4B).T2WIcanbestdetectzonalanatomicalanomaliesinPCacells (or to depict seminal vesicle invasion and extracapsular extension of disease). Normal PZtissueiswater-richwithmany ductalandacinarcomponentsandsparselyinterlacedsmoothmuscle.T2-weighted picturesshowitasbright.PCainthePZ showsasaroundedorill-definedlow-signalintensityfocus(Fig.4A),unlikethelooselypackedtypicalPZtissue.Prostatitis, atrophy,andpreviousbiopsy-relatedhaemorrhagescanmimicalow-signalintensityfocusinthePZofT2-weightedimages. On T2WI, normalTZ tissuelooksdarkerthanthePZdue to its lower watercontent,compact smooth muscle,and sparser glandularcomponents.PCaintheTZappearsasauniform,low-signalmasswithfuzzyborders.Duetoitslargemuscleand fibrouscontent,theTZmaylooklow-signalintensity,makingitdifficulttoidentifycancerfromstromalBPH.[24]DWI.PCa has dense tumor cell areas. The high intracellular/extracellular volume ratio of these locations limits water molecule Brownianmotionintheextracellularspace.DWIdetectswatermolecules'randomBrownianmotion.Changingmagneticfield durationand strength yields two or more DWI pictures (indicated by a b-value). Cancer appears bright hyperintense on DW imaging because restricted water diffusion reduces signal loss. Cancers appear as hypointense dark spots on an Apparent DiffusionCoefficient(ADC) mapmadefromnumerousb-valueDWimages(Fig.4C).ADCreadingspredict cancer aggressiveness.Ultra-highb-valueDWI(e.g.,2000seconds/mm2;Fig.4D)mayimproveindexlesiondetermination.

e-ISSN:2395-0056
Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
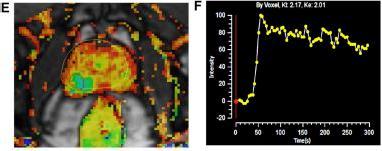
Fig – 4 : The mpMRI depiction of right posterolateral lesion: (A) axial T2-weighted image with lesion inred outline, (B) 3D T2-weighted view of prostate contour (brown) and lesion (red), (C) ADC image with lesion in red outline, (D) computed high b-value = 2000 seconds/mm2 image with lesion in red outline, (E) dynamic contrast-enhanced pharmacokinetic map with lesion in red outline, (F) average time–signal intensity curve plot of the lesion, and (G) PIRADS version 2 location of lesion (orange). Dynamic contrast-enhanced imaging. DCE-MRI uses 3D T1-weighted images before, during, and after intravenous contrast media injection (typically low-molecular-weight Gadolinium chelates that rapidly diffuse in extravascular extracellular space). As said, aggressive tumors produce angiogenic agents that stimulate microvessel proliferation. New microvesselsaredisorderedandleakyduetoweakenedwalls.TumorangiogenesiscausesearlyDCE-MRenhancementinPCa tissue.TheDCE-MRimagesareusuallyanalyzedforlesions(a)qualitatively,byvisual inspection of subtraction time points forpotentiallycancerousspotsshowingfocalenhancement;(b)semiquantitatively,bytime–signalintensitycurveanalysis (Fig. 4F) of suspicious voxels to determine parameters like time-to-peak,wash-inslope, etc.; and/or(c) quantitatively,by compartmentalpharmacokineticmodellingthatusescontrastmediaconcentrationandotherparameters(Fig.4E).
Magnetic resonance spectroscopic imaging. MRSImeasuresprostatetissuecellularmetaboliteconcentrations. Citrate is highinhealthyprostatetissue,especiallyinthePZ.Canceroustissuelowersthem.PCaalsoincreases cholinelevelsdueto celldensity,cellmembraneturnover,andphospholipidmetabolism.MRspectroscopy distinguishesPCatissuebyitshigh choline-to-citrateratio.Becausecreatineandcholinehavesimilarresonant peaks,theratioofcholine+creatinetocitrate is usually employed. Turkbey et al. found that MRSI enhances mpMRI-based PCa detection. [25] MRSI takes longer and demands technological skill. Its clinical use for PCa diagnosis is limited. It generally stages and detects radiation recurrence.
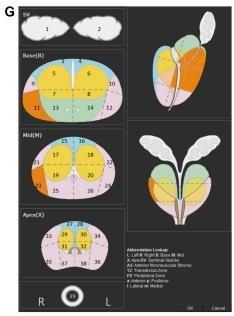
mpMRI-detected PCa mpMRI-basedPCadetectionissuccessful.Rais-Bahramietal.discoveredthatbiparametricMRI(T2WI +DWI)hadanAUCof0.8forPCadetectionin143men(whichoutperformedtheAUCsof0.66and0.74forPSAlevelandPSA density,respectively).Schootsetal.meta-analyzedthediagnosticbenefitsofmpMRI-targeted biopsiescompared.systematic biopsies.MRI-guidedbiopsiesdetectedclinicallysignificantcancer(91%vs.76%)andclinicallyinsignificantcancer(44%vs. 83%)betterthanTGBs.Panebiancoetal.randomlyassigned1140malestoeitherstandardTGBsormpMRI+TGBs.Thefirst cohortdetected38%andthesecond72%.NomenwithnegativeMRIsdevelopedclinicallysignificantcanceronsaturation biopsies. Targeting MRI-suspicious lesions isbeneficialforbiopsy-naïve,previousnegative,andactivesurveillancepatients. Thescanningacquisitionprocedure and scan reading accuracy are crucial to mpMRI-based PCa detection. Standardizing prostate mpMR imaging acquisition and reading is underway. The European Society of Urogenital Radiology (ESUR) publishedtheProstateImagingandReportingDataSystem(PIRADS)in2012tostandardizempMRIlesionacquisitionand cancersuspicionlevelandsiteassessment.PCadetectionincreaseswithPIRADSsuspicionlevelinseveraltrials.Recently,the

e-ISSN:2395-0056
Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
American College of Radiology, ESUR, and AdMeTech foundation worked to upgrade this standard to PIRADS version2, whichamongotherchangesalsopromotestheuseof39-regioncharts(Fig.4G)todefinelesionlocations. Accordingtothe newestPIRADSversion2,DCE-MRIdoesnothelptotheoverallassessmentofsuspicionlevelforlowergrade(PIRADSlevels 1and2)andhighergradelesions(PIRADS4or5).(PIRADS4or5).ApositiveDCE-MRI raisesPIRADSlevel4suspicionfor equivocalormoderate-gradePIRADS3lesions.Hence,themainstreamexpertopinioncurrentlyconsidersdiagnosticquality T2WIandDWI/ADCimagestobethemajorMRIsequencesusedfor suspicion levelassessment of lesions. DCE-MRI is still suggestedtodetecttinyclinicallyimportanttumors.
MRI–US fusion.
ThissectiondiscussesanewPCaimagingmethodthatcombinesUSandMRI.Fusionimagingcombinesthe advantagesof USandMRimagingwithoutcompromisingtheirdiagnosticclinicalrelevance.mpMRI'sclinicallysubstantialPCadetection sensitivityandspecificityhavetransformedPCadiagnosis.Hambrocketal.comparedin-borempMRI-guidedbiopsiesto10coresystematicTRUSbiopsiesinmenbeforeRP.MRI-guidedbiopsiesdetectedPCa88%betterthangrayscaleTGBs(55%;Pvalue 0.001). MRI is accurate, but given the number of prostate biopsies conducted each year, it is too expensive and impracticaltoutilizealone(approximatelyamillionintheUSalone).In-borebiopsiestakelongerwithoutreal-timeimaging, makingpatientsuncomfortable.Duetotheintense magneticfield,safetyrequiresspecialistequipmentandneedles.In-bore biopsieswithreal-timeimagingandfasterroboticneedleplacementsarebeingdeveloped.[26-27]GrayscaleUSimagingis cheaper and faster than in-bore MRI, although it may not be as diagnostic. mpMRI–USfusion-guided biopsies are now a feasible alternativetoMRI- guided, inbore, and conventional TGBs. MRI–US fusion-guided biopsies are conducted in an outpatient clinicwith liveB-modeUS,reducing expenses and treatment time.Toidentifyaberrant MRI areas,thepatient undergoesanmpMRIpelvicexaminationbeforetheoperation.Thebiopsyproceduretargetsthesesitesbymappingthemonto USimagesviaimagefusionorregistration.ExperturoradiologistscancognitivelyorvisuallyfuseMRIandUSpictures.Thisis subjective and may require knowledge only found in huge academic research institutions. Regulatory- cleared, commerciallyavailableMRI–USfusiondevicesaregrowingmorepopular.Real-timeUSbiopsiesofvirtual MRI targets are easier with fusion platforms. The platforms differ in US capture (3D volumetric, 2D sweep, etc.),biopsy targeting (e.g., electromagneticandelectromechanical),biopsyroute(transrectalvs.transperineal),and imagefusiontechnique(rigidvs. elastic).Mostsystems storebiopsied sites withabnormalgradinganduseMRI/US fusion-based targeting guidance.Active surveillanceandtargetedtherapydependonaccuratebiopsyplacements;therefore,thishasmajorimplicationsforpatients. Three widely investigated fusion biopsy platforms Artemis (Eigen), UroNav (Invivo/Philips), and Urostation will be discussed here(Koelis).BiopSee,VirtualNavigator,HI RVS,BioJet,MonaLisa,andLOGIQ9(MedCom,Esaote,Hitachi, and GeoScan)areotherplatforms(GEHealthcare).
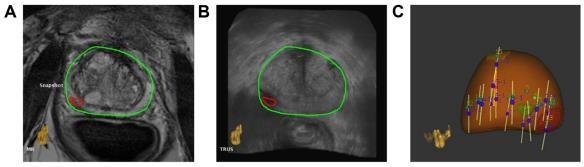
Artemis. Eigen,USA,makesArtemis.SemiroboticmechanicalstabilityoftheTRUSprobeduringbiopsyreducesfree-hand distortion. ProFuse radiology software annotates worrisome lesions in 3D mpMR images before the surgery.The TRUS probemechanicallyrotatestoacquire3DvolumetricUSdata.This3DUSvolumeisrigidlyandelasticallymergedwiththe3D MRI volume(Fig.5A and 5B).Rigid fusioncorrects orientationvariations between 3D MRI and 3D US volumes, while elastic fusion accounts for local shape deformations caused by patientorientation,bladder/rectalfilling,andendorectal coilorTRUSprobepressure.FusiontransfersvirtualMRIlesion sitestoreal-timeTRUSimagesfortargeting.Afterfusion,a visiblegraphicalinterfacebasedonelectromechanical tracking and shot sites moves the probe/needle guide assembly to lesion spots (Fig. 5C). MRI–US fusion-based targeting and automatic template distribution of systematic, random biopsy cores based on prostate formand volumeareavailable. The Artemis system revealedcancer in53%of a mixedgroupof 171 men on active surveillance and earlier negative biopsies in a Sonn et al. investigation. Fusion biopsy-guided cores detectedthree timesmore malignancythansystematic randombiopsiesand more clinicallyrelevanttumors(P = 0.001). ThebiopsyfindingscorrespondedwithmpMRIsuspicionlevelofthetargetedlesions,andmenwiththehighest suspicion level had a 94% cancer detection rate. [28] Sonn et al. revealed 34% of prior negative men got PCa, 72% of whom had clinically severe illness. Fusion biopsies found 1.4 times but 15% as many unimportant tumors, accordingtoSonnetal. Wysocketal.comparedArtemis-targetedandexpertcognitivefusionbiopsiesin125menwith172MRI-suspiciouslesions. Artemistargetedfusionbiopsiesfound20.3%clinicallysignificanttumorspertargetcomparedto15.1%utilizingcognitive targeting(P=0.0523).Device-targetedbiopsywasmorepathologicallyinformativethancognitivebiopsies(P=0.0104).

e-ISSN:2395-0056
Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
UroNav. Invivo/PhilipsmarketstheUroNavplatform,createdattheNationalInstitutesofHealth,Bethesda,USA.Anexternal electromagneticfieldgeneratortracks andguidesneedlebiopsies transrectally. DynaCADforProstate marks problematic lesions on preprocessed 3D mpMR images. A 2D freehand sweep from prostate base to apexacquiresthe3DUSvolume duringbiopsy.A stiffmethodthataccounts forrotationalandtranslationalvariancescan fuse3D MRIand 3D US pictures. VisuallyadjustingtheUSprobepressureontheprostatecanchangethedistortionbetweentheUSandMR3Dpictures.After fusion,electromagneticguidanceguidestheprobe/needleassemblytovirtualMRIlesions.Pintoetal.discoveredPCain28%, 69%, and 90% of 101 patients using the UroNav platform (P< 0.0001). In their investigation, the number of mpMRI sequences (T2WI, DCE, DWI, and MRSI) that detected a lesion as positive low (2 or less), moderate (3), or high (4) determined its suspicion grade and the chance of canceruponbiopsy.Vourgantietal.foundPCain37%of195menwith priornegativebiopsies,and fusion-guided biopsies found high-grade malignancy in all males (n = 21), while systematic biopsies missed them in 12 individuals.[29] Siddiqui et al.discovered that targeted biopsy revealed 30%morehigh-risk cancer (P < 0.001) and 17% fewer low-risk cancer (P< 0.001). Targeted biopsies outperformed systematic biopsies in predictinglow-riskcancerin170menfollowingRP(P<0.05).
Urostation. Koelis, France's Urostation platform uses software image registration instead of electromechanical or electromagnetic tracking like Artemis and UroNav. A 3D US probe stitches together an initial volume. This 3D volume marksconsecutivebiopsyspots.TheUSvolumeiselasticallymergedwith3DMRimagestomapMRIlesions.Then,theUS probeisfreehand-manoeuvredtothelesionareasforbiopsy.A3Dvolumeistakenwiththeneedleinplaceandelastically fused with the initial 3D reference volume to confirm each biopsy location. Using fusion-guided biopsy, Ukimura et al. targeted MR-visible, hypoechoic, and isoechoic lesions on a phantom withthe Urostation platform.84% offusion-guided biopsieshitlesions.Inaretrospectiveinvestigationof90patients,Rud et al found PCa diagnosis rates of 10%, 27%, and 91%forlow-,medium-,andhigh-MRIsuspicionlevels.Fusionbiopsiesdetectedmoreclinicallyseriouscancerin152men thansystematicbiopsies(P=0.03).
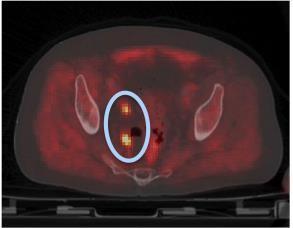
PET Imaging. Gammacameras imageintravenousradiolabeledtracers intheprostateduringPET.Itisutilizedfor cancer staging, biochemical failurefollowing radiation, and lymphnode metastases (Fig.6). PET/MRIorPET/CTis utilized with anatomical imaging to show prostate cell metabolic, molecular, or cellular activity. The tracer and biological process (metabolism,cellulargrowth,receptorbinding)determinePETimagingmodalities.

e-ISSN:2395-0056
Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
– 6: PET/CT image postprostatectomy depicting possible lymph node metastasis (blue oval withorange hot spots)
Metabolism-focused. 18F-fluorodeoxyglucose. The Warburg impact leading the charge glycolytic pathway glucose metabolism in malignant tissue than normal tissue. 18F-fluorodeoxyglucose is the most common tumor glucose metabolismradiotracer(18F-FDG).18F-FDGcannotdetectearlyorrecurringPCaduetoinadequateglucosemetabolismin small,developingPCacellsandtheprostate'scloseproximitytotheurinebladder,whichconfounds uptakedata.Tumorcells withinadequateglucosemetabolismhavelowradiotraceruptakeand overlapwithnormal tissueand BPH.Prostatitis may ingestmoreFDGthanPCacells.Yangetal.studied100patientswithincidentalFDGuptakeandfoundthat20hadmalignant lesionsand80hadbenignlesions.InPSArecurrencepatientswithnegativewhole-bodybonescans,FDGPETimagingmay stillrevealpelviclymphnodemetastases.Inanearlyinvestigationof24menwithnegativebonescans,Changetal.reported FDG-PET imaging to have 75% sensitivity, 100% specificity, 100% PPV, and 67.7% NPV for detecting metastatic pelvic lymphnodes.
Proliferation 1-Amino-3-fluorine-18-fluorocyclobutane-1-carboxylic acid. PCa cells upregulate amino acid transport, whichtheradiotracer18-FACBCexploits.Thisradiotracer'slowurinaryexcretionimproves18-FACBC uptakedetectionin malignant tumor cells. [30] Schuster et al. found that 18-F ACBC PET detected recurrent prostatecancermoresensitively (89%)thanFDA-approved[111In]capromabpendetideSPECT/CTorProstaScint (69%). Schuster et al. additionally found that the highest standardized absorption value of anti-18-F ACBC associated with Gleason score at all time points (P < 0.05) in PCa prostatectomy specimens. [31] Turkbey et al. examined 21 patients before prostatectomy with 18-F ACBC PET/CTand3TmpMR.18-FACBCPET/CTdemonstratedasensitivityof90%fordominantPCaand67%and66%forPCa, respectively,comparedtohistopathologicresults.BPHoverlappedwithcancertissue'sincreasedtraceruptake.T2-weighted MRIwith18-FACBChadatumorlocalizationPPVof82%,greaterthaneithermodalityalone.11C-Choline,18F-fluorocholine. PCa increasesmembraneproduction.Cholinetransportersbringcholineintocellstosynthesizephosphatidylcholine,a cell membranecomponent.Indexcancersingest11C-cholineand18F-fluorocholine(FCH)radionuclidesfortracerimaging.Kwee et al. performed FCH PET/CT in 50 patients with increased PSA levels after treatment (RP, RT, brachytherapy). 88% of patientswithaPSA<1.1ng/mLhadabnormaltumoruptake,comparedto6%belowthisthreshold.Thus,imagingPSAlevels affectedFCHPET/abilityCT'stoidentifyPCarecurrence.Simoneetal.assessedbiochemicalrecurrencein146patientswith low PSA (<1 ng/mL) using a new FCH PET/CT imaging acquisition strategywithanearlydynamicphase.InthislowPSA cohort,FCHPET/CThadasensitivityof79%,suggestingitcoulddetectPCarecurrenceearly.]Kitajimaetal.compared11Ccholine PET/CT to mpMRI in 115 prostatectomy patients. mpMRI outperformed 11C-choline PET/CT for recurrence diagnosis (AUC 0.909 vs. 0.761, P-value 0.01). However, 11C-choline PET/CT outperformed mpMRI for lymph node metastatic identification. A meta-analysis of 3167 individuals from 47 studies found 11C-choline or 18F-FCH PET/CT beneficialasafirstimagingscanforPCapatientswithbiochemicalrecurrenceandPSAvaluesbetween1and50ng/mL.
Receptor-targeted PSMA.PSMAisamembraneglycoproteinhavingalargeextracellular,transmembrane,andintracellular domain.AllstagesofPCasubstantiallyupregulateprostaticepithelialcellPSMA.[32]PSMAexpressionincreases with tumor aggressiveness, metastasis, and disease recurrence, making it a rational target for ligand– receptor-based imaging and therapy.Radiolabeled antibodies/antibodyfragments havetargetedintracellular or extracellularantigenmotifsinanimal

e-ISSN:2395-0056
Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
models andpilothuman trials.64Cu-labeled aptamersand11C-,18F-,68Ga-, and86Y-labeledlow-molecular-weightPSMA inhibitors are PSMA-based radiotracers.In initial clinical trials, 68Gal- labeled PSMA inhibitor detected PCa relapse and metastasesbetterthan18F-choline.[33]Indium-111radiolabelingofthe7E11C5.3antibodyproducedProstaScintscan,the only FDA-approved PSMA agent. ProstaScint scan can describe recurrence in patients with increased serum PSA levels followingprimarytherapybyidentifyingtheintracellularpartofPSMA.Preoperativelymphnodestagingusing68Ga-PSMAPET outperformed CT andMRI in 130 intermediate- to high-risk PCa patients. Ga-PSMA has also been demonstrated to restagePCainsalvageRTcandidateswithPSAlevels<0.5ng/mL.
Hodge et al. pioneered prostate transrectal biopsies using grayscale B-mode US imaging in 1989. Transrectal USswiftly replacedphysicalDREforguidingneedlebiopsiesforPCadiagnosis.Today,imagingisessentialforPCa management.US, MRI,andPETareusedtodetectandlocalizePCa.B-modeorgrayscaleUSisstillthemost extensivelyusedPCadetection modality, although new scientific data are highlighting additional promising modalities. Previously utilized mainly for stagingandrecurrencedetection,clinicsworldwidearenowusingmpMRI for PCa detection via direct in-bore MRI-guided biopsy or MRI–US fusion-guided biopsy. CEUS and RTE have become promising US-guided PCa detection methods. This suggests a promising future for an integrated multimodality (mpUS and mpMRI) PCa detection strategy. PET imaging for PCa is useful for cancer staging, biochemical failure after radiation, and restricted diagnosis. Tumor biology and evolution are complex. To choose the best imaging method, one must understand tumor growth mechanisms. Figure 2 summarizes the tumor traits that imaging methods exploit. Grayscale or B-mode US modalities image zonal anatomical deficitsproducedby prostaticcancers.DopplerUSandCEUSimagetumormacrovascularityandmicrovascularitybecause tumorsareangiogenic.ProstateRTEreliesonrigidPCa tissue.mpUSormpUS-basedimagingofPCausesanatomicaland functionalUS imaging techniques.Researchers worldwidearestudying this.Standardizing intraprocedurempUS imaging andlesionassessmentmayenableaccurate,cost-effectiveoffice-basedprostatebiopsies.
PCaimaginghasbeenmostaffectedbympMRIinrecentyears.SeveralstudieshaveshownthatdirectMRI-guidedbiopsiesor MRI–US fusion-guided biopsies increaseclinically important cancer detection and decrease clinically insignificant cancer detection.Thispreventsunderdetectionofmalignantdiseaseandoverdetection(and overtreatment)ofindolentdisease, whichaffectsPCacare.mpMRIdetectsPCausinganatomicalandfunctionalmethods.T2-weightedimagesshowsuspicious anatomical variations in normal prostate tissue, which may be causedbycancer;DWImeasurestherestrictedBrownian motion of water molecules caused byincreased cellularity of tumors; and dynamic contrast-enhanced imaging exploits angiogenesis-induced tumor vascularity. MR spectroscopic imaging can also detect prostate cancer-induced metabolite alterations. PET imaging helps detectbiochemicalrelapse,PCarecurrence,andmetastases.Thisusesseveralradiolabeled tracers. PET is also used with CT or MR to locate radiotracer uptake hot spots. Tumor biology determines radiotracer selection.The18F-FDGtracertargetstumorglucosemetabolism,the18FACBC-,choline-,andacetate-basedtracerstarget moleculeselevatedandproliferatingduringtumorgrowth,andthePSMA-basedtracerstargetligand–receptorinteractionsin tumors with enhanced PSMA expression. In conclusion, cutting-edge imaging techniques have transformed PCa management,andmoreinterestingresearchandadvancementsawait.
Imagingisbecominganincreasinglysignificanttoolforboththeearlydetectionofprostatecancerandthe managementof thedisease.Thisarticleprovidesasummaryofthemostimportantimagingmodalitiesthatare employedinthediagnosis and localization of prostate cancer, including multiparametric ultrasound, multiparametric magnetic resonanceimaging, MRI–USfusionimaging,andPETimaging.Thebiologicalaspectsof tumors,whichareresponsibleforthejustificationofthe applicationofparticularimagingmodalities,aregivenalotofattention.
[1] Jemal,R.Siegel,E.Ward,T.Murray,J.Xu,andM.J.Thun,“Cancerstatistics,2007,”CA:ACancerJournalforClinicians, vol. 57,no.1,pp.43–66,2007.
[2] E. D. Crawford and A. B. Barqawi, “Targeted focal therapy: a minimally invasive ablation technique for earlyprostate cancer,”Oncology,vol.21,no.1,pp.27–32,2007.
[3] M.R.Cooperberg,J.W.Moul,andP.R.Carroll,“Thechangingfaceofprostatecancer,”JournalofClinical Oncology,vol. 23,no.32,pp.8146–8151,2005.
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
[4] V.Scattoni,A.Zlotta,R.Montironi,C.Schulman,P.Rigatti,andF.Montorsi,“Extendedandsaturationprostaticbiopsy in thediagnosis andcharacterizationof prostate cancer: a critical analysisofthe literature,”European Urology, vol. 52, pp.1309–1322,2007.
[5] Ohori M, Wheeler TM, Dunn JK, Stamey TA, Scardino PT. The pathological features and prognosis of prostatecancer detectablewithcurrentdiagnostictests.JUrol.1994;152(5pt2):1714–1720.
[6] HodgeKK,McNealJE,StameyTA.Ultrasoundguidedtransrectalcorebiopsiesofthepalpablyabnormalprostate.J Urol.1989;142(1):66–70.
[7] HodgeKK,McNealJE,TerrisMK,StameyTA.Randomsystematicversusdirectedultrasoundguidedtransrectalcore biopsiesoftheprostate.JUrol.1989;142(1):71–74.8
[8] BjurlinMA,WysockJS,TanejaSS.Optimizationofprostatebiopsy:reviewoftechniqueandcomplications.UrolClinNorth Am.2014;41(2):299–313.
[9] SinghH,CantoEI,ShariatSF,etal.Predictorsofprostatecancerafterinitialnegativesystematic12corebiopsy.JUrol. 2004;171(5):1850–1854.
[10]TairaAV,MerrickGS,GalbreathRW,etal.Performanceoftransperinealtemplate-guidedmappingbiopsyindetecting prostatecancerintheinitialandrepeatbiopsysetting.ProstateCancerProstaticDis.2010;13(1):71–77.
[11]ZaytounOM,MoussaAS,GaoT,FareedK,JonesJS.Officebasedtransrectalsaturationbiopsyimprovesprostatecancer detectioncomparedtoextendedbiopsyintherepeatbiopsypopulation.JUrol.2011;186(3):850–854.
[12]IsariyawongseBK,SunL,BanezLL,etal.SignificantdiscrepanciesbetweendiagnosticandpathologicGleasonsumsin prostatecancer:thepredictiveroleofageandprostate-specificantigen.Urology.2008;72(4):882–886.
[13]Braeckman J, Autier P, Soviany C, et al. The accuracy of transrectal ultrasonography supplemented with computer-aidedultrasonographyfordetectingsmallprostatecancers.BJUInt.2008;102(11):1560–1565.
[14]SimmonsLA,AutierP,Zat’uraF,etal.Detection,localizationandcharacterisationofprostatecancerbyprostate HistoScanning(TM).BJUInt.2012;110(1):28–35.
[15]SchiffmannJ,MankaL,BoehmK,etal.ControversialevidencefortheuseofHistoScanninginthedetectionofprostate cancer.WorldJUrol.2015;33(12):1993–1999.
[16]LochT.Computerizedtransrectalultrasound(C-TRUS)oftheprostate:detectionofcancerinpatientswithmultiple negativesystematicrandombiopsies.WorldJUrol.2007;25(4):375–380.
[17]HalpernEJ,FrauscherF,StrupSE,NazarianLN,O’KaneP,GomellaLG.Prostate:high-frequencyDopplerUSimagingfor cancerdetection.Radiology.2002;225(1):71–77.
[18]SauvainJL,SauvainE,RohmerP,etal.ValueoftransrectalpowerDopplersonographyinthedetectionoflow-risk prostatecancers.DiagnIntervImaging.2013;94(1):60–67.
[19]PostemaAW,FrinkingPJ,SmeengeM,etal.Dynamiccontrast-enhancedultrasoundparametricimagingforthedetection ofprostatecancer.BJUInt.2015Mar6.doi:10.1111/bju.13116.
[20]GoodDW,StewartGD,HammerS,etal.Elasticityasabiomarkerforprostatecancer:asystematicreview.BJUInt. 2014;113(4):523–534.
[21]ZhangB,MaX,ZhanW,etal.Real-timeelastographyinthediagnosisofpatientssuspectedofhavingprostatecancer:a meta-analysis.UltrasoundMedBiol.2014;40(7):1400–1407.
[22]ZhaiL,MaddenJ,FooWC,etal.Acousticradiationforceimpulseimagingofhumanprostatesexvivo. UltrasoundMed Biol.2010;36(4):576–588.
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 12 | Dec 2022 www.irjet.net p-ISSN:2395-0072
[23]Boehm K, Salomon G, Beyer B, et al. Shear wave elastography for localization of prostate cancer lesions and assessment of elasticity thresholds: implications for targeted biopsies and active surveillance protocols. J Urol. 2015;193(3):794–800.
[24]BhavsarA,VermaS.Anatomicimagingoftheprostate.BiomedResInt.2014;2014:9.
[25]Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection histopathologiccorrelation.Radiology.2010;255(1):89–99.
[26]Tokuda J, Tuncali K, Iordachita I, et al. In-bore setup and software for 3T MRIguided transperineal prostate biopsy. PhysMedBiol.2012;57(18):5823–5840.
[27]Tilak G, Tuncali K, Song SE, et al. 3T MR-guided in-bore transperineal prostate biopsy: a comparison of roboticand manualneedle-guidancetemplates.JMagnResonImaging.2015;42(1):63–71.
[28]Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magneticresonanceultrasoundfusiondevice.JUrol.2013;189(1):86–91.
[29]VourgantiS,RastinehadA,YerramNK,etal.Multiparametricmagneticresonanceimagingandultrasoundfusionbiopsy detectprostatecancerinpatientswithpriornegativetransrectalultrasoundbiopsies.JUrol.2012;188(6):2152–2157
[30]Marko J, GouldCF, Bonavia GH, Wolfman DJ. State-of-the-artimagingof prostatecancer. Urol Oncol. 2015Jun15. pii: S1078-1439(15)00239-2.doi:10.1016/j.urolonc.2015.05.015.
[31]SchusterDM,TaleghaniPA,NiehPT,etal.Characterizationofprimaryprostatecarcinoma byanti-1-amino-2-[(18)F]fluorocyclobutane-1-carboxylicacid(anti-3-[(18)F]FACBC)uptake.AmJNuclMedMolImaging.2013;3(1):85–96.
[32]Bouchelouche K, Turkbey B, Choyke PL. Advances in imaging modalities in prostate cancer. Curr Opin Oncol. 2015;27(3):224–231.
[33]Afshar-OromiehA,ZechmannCM,MalcherA,etal.ComparisonofPETimagingwitha(68)Ga-labelledPSMAligand and (18)F-choline-based PET/ CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41(1):11–20