International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 11| Nov 2022 www.irjet.net p-ISSN: 2395-0072
Characteristic manifestations of uranium enrichment in certain PanAfrican granitoids of NW Karbi Hills, Assam (NE India)
Himsekhar Borthakur1, Dilip Majumdar2 and Debasish Borah31 Research Scholar, Dept. of Applied Geology, Dibrugarh University, Assam (India) 2 Professor, Dept. of Applied Geology, Dibrugarh University, Assam (India)
3 Dept. of Applied Geology, Dibrugarh University, Assam (India) ***
Abstract-The northwestern edge of KarbiHills witnessed massivegranitoidsformationduring Cambro-Ordoviciantime forming a part of late Pan-African magmato-orogeny. The mediumtofine-grained,leucocratic,non-porphyriticgranitoids contain mafic xenoliths, aplities, microgranite, and quartz veins, invariably saturated with either REE or U and Th, found in Parkop Pahar (PKP), Gufa Pahar (GP), Pulibagan (PULI), and Udmarigaon (UDG) granites. Thin section petrography, supported by powder XRD, reveals a distinct assemblage of U and Th bearing accessory minerals such as uraninite, brannerite, coffinite, thorite, and others, with U and Th concentrations of up to 159 and 121 ppm, respectively. The presence of pitted surfaces in plagioclase and quartz with occasional disseminated alpha tracks, pleochroic haloes in biotites, and/or apatite inclusions in biotites is a miniature scale-modeloftheregionalhydrothermalalterationtypesand patterns representing uranium mineralization/mobilization. Plagioclase feldspar is the most vulnerable to radiation damageanddamage-controlledfluid-assistedalteration,which may redistribute metals, including actinides, in which an alteration sequence Na+ > K+ > H+ has been proposed, integrating U and REE redistribution. The alteration process also included interaction of the hydrothermal fluid with primary U-bearing minerals, inclusions resulting in uraninite resorption, redistributionofelements, includingUandPb, and resetting of isotopic make-up, resulting in the characteristic background ionizing radiation (BIR) profile. The record of aquatic BIR (0.016-0.059) mR/h, total dissolved solids (TDS) values (900-6700) mg/l, and marginal aquatic uranium concentration (<1ppb) establishes the reducing condition of U6+ and formation of low soluble U4+minerals with an increase in daughter element concentration of U decay series supported by low pH of water (5.46-5.76).
Key words: Karbi Hills, non-porphyritic granitoids, BIR, TDS, pH.
1. INTRODUCTION
KarbiHillsisunexploredforuraniumreserves,althoughit isreportedfromtheMeghalayapartoftheShillongplateau. TheA2 typeNWKarbiHill’sgraniteswereformedduring post-collisionalriftingepisode,oftenrichinREEand/orUTh bearing accessory minerals. Geochemical attribute suggestsmetaluminoustoperaluminouschemistry,A2type, within platecharacter(MajumdarandDutta,2016[1]).The mineral composition is dominated by quartz, feldspar,
plagioclase, and biotite, with a significant amount of accessoryphasessuchasapatite,zircon,xenotime,monazite, andallanite.Thepetrographyofthestudiedgranitesreveals radioactivity damages such as pitted quartz and feldspar surfaces, pleochroic haloes in biotites, the presence of metamictallanite,andalphatracksonmineralsurfaces.The U and Th bearing accessory minerals such as uraninite, brannerite, coffinite, thorite and their alteration products promotesanoverallincreaseinUand/orThconcentration up to 45 ppm (Majumdar and Dutta, 2016). There are reports on a related study on outdoor and indoor background ionizing radiation (BIR) (10.52 mBq/kg/h to 56.29 mBq/kg/h approx.) as well as the determination of radonanduraniumcountsinresidualsoilandnaturalwater in Karbi Anglong (Kakati and Bhattacharjee, 2011[2]). Accordingtoreports,radioactivitycausedbythoriumand uranium has been noted in granitoids terrain of Bargaon, Donkamokam, and Teragaon (Karbi- Hills). (GSI Report, 2009). Radionuclides, which act as contaminants, are mobilisedbygroundwaterornaturalwatersourcesfoundin granite outcrops (Ahmed et al., 2018[3]). According to Kozowskaetal.,(2005)[4],highTDSbearingnaturalwater is being polluted by dissolved radio nuclides, which are progenies of Th and U decay series derived from various geologicalstrata.The pHofwaterissaidtocontroluranium mobility. Hexavalent uranium predominates at low pH (Himrietal.,2000[5]).NaturalwaterswithapHlessthan7 areconsideredacidicduetoalackoforlowconcentrationof calciteanditscloserelationshipwithgraniticrocks(Robins, 2002[6]). The interaction of water and acidic (granite) catchment is the primary cause of the low pH level of the naturalwater.
This paper discusses the issue of high background ionizing radiation (BIR), total dissolved solids (TDS), and aquaticuraniumconcentration,pHdependenceonUandTh concentration,andgeochemicalbasisinthegraniticdomain ofPan-Africanmagmato-orogenicgranitesintheNWKarbi Hills,Assam.
2. METHODOLOGY
InthenorthwestoftheKarbiHills,bothterrestrialand aquaticradiationswererecordedusingGammadosimeter RadexRD1503+.Thisextremelyaccurateandtrustworthy dosimetercanmeasurein-situgammaradiation.Thedevice
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 11| Nov 2022 www.irjet.net p-ISSN: 2395-0072
measurestheBIRvaluesinunitsofSv/yormR/hr.Untilthe iconsquareinthedigitaldisplayhascompletedfourcycles offourminutesandsixtyseconds,theBIRismeasured.This radioactive monitor, Radex RD1503+, calculates and evaluates the amount of dose rate of gamma radiation receivedoutsideorbyapersonexposedtoradiationinthe environment while also taking into account the contaminationofobjectsbysourcesofbetaparticlesorX-ray emissions. Aquatic radiation recordings are measured primarilyatdischarginglocationsofnaturalwater.
A portable MODENNA digital pH meter was used to measure the pH level of the natural water sources in the studyarea.Theelectrodeiscalibratedusingbuffersolutions withpH7,4,and10beforebeingusedinthefield,anditis strictly required to rinse and dry the electrode with distil waterordeionizedwaterinbetweeneachdifferentsolution immersion.Thus,during fielduse,the pH meter electrode was routinely rinsed with distilled water, sanitised, and driedwithtissuepaperfollowingeachpHreading.
Total dissolve solids measurement(TDS) was done using SEMCO India’s water and soil analysis kit. Following the calibration process the TDS readings were eventually recordedafterremovingtheelectrodefromthedistilwater anddippingitintothebottlescontainingthecollectedwater sample.
The laboratory study including the petrography observationsandXRDweredonesoonafterthecompletion offieldwork.Thinrectangularchipsofrockmeasuring420.5 cubic cm were cut out of granitoid samples using the inhouse facility of the Department of Applied Geology, DibrugarhUniversity.Thesechipswerebeingdeliveredto ONGC geological Laboratory in Sibsagarh, Assam, and Continental Instruments in Lucknow for treatment to a polishedthinsection.Thinsectionswereobservedusingthe polarizingmicroscopeOlympusBX51.
AquickanalyticalmethodcalledX-raypowderdiffraction (XRD)isgenerallyusedtodeterminethephaseofcrystalline materials. In an XRD study, the sample to be examined is homogenisedandcoarselypulverisedtoameshsizeof170 ASTMbeforetheaveragebulkcompositioniscollected.The Powder X-ray Diffractometer, Rigaku Ultima IV, was used withthehelpoftheCentralSophisticatedInstrumentation Centre (CSIC), Dibrugarh University. It is equipped with a coppertubewithawavelengthof1.5418oAandadetector withaxenonfilledsealedproportionalcounter.
3. GEOLOGICAL SETUP
TheMeghalayaCratonandtheKarbiHillsareseparatedby the Kopili lineament, often described as the "twin cratons within the Shillong Plateau" (Murlidharan and Desraj, 1983[7]; Bora and Roy, 1999[8]). Both are made up of Proterozoic basement rocks that have been penetrated by magmaticintrusionswithvariousgeneses,anditiscovered
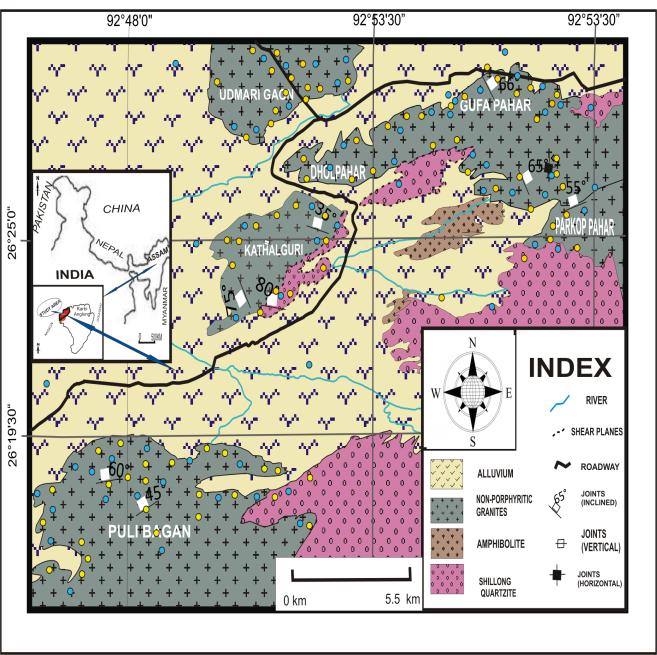
with Proterozoic and Tertiary sediments. The intrusive/extrusivebodiescontainatleastfiveepisodesof magmatic activity, including an early phase of basaltic composition,thesecondandthirdphasesoflargebatholiths of porphyritic granitoids, the fourth phase of small-scale intrusionsofdoleritesandtrapsknownastheMikirTraps, andalatephaseofalkaline-carbonatiteformation.Thestudy domain (Fig.1) carries evidences of evidences like flow banding, breccias with chilled margins having necessary indicationsofviolentintrusionthroughcoldercrustinpost collision extensional settings. The Pan-African episode leadingtomassivegranitoidsformationduringPan-African episodewasasuddenandshortlivedphenomenon,causing invasionofmagmathroughgneissicbasementcomplex,older amphibolites and Shillong Group of supracrustals. These granitoids carry evidences of true volcanic domain, where thegranitoidsbeardegassingvents,vesiclesoccasionallyat theirapophyses,eitherREEorUandThenriched.
Fig-1: GeologicalMapofStudyArea
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 11| Nov 2022 www.irjet.net p-ISSN: 2395-0072
(Fig.2d)likesericitisationandkaolinizationhavebeennoted inthesegranites,markingthealterationsequenceNa+ > K+ >H+
Fig-2: PhotomicrographsofgranitesofNWKarbiHills showinga)PleotropicHaloinBiotiteb)Metamictisationof Allanitec)MyrmekiticTextured)Alterationtexture observeduponplagioclasegrains(sericitisation)e)Alpha tracks

4. RESULT AND DISCUSSION
4.1 Petrography
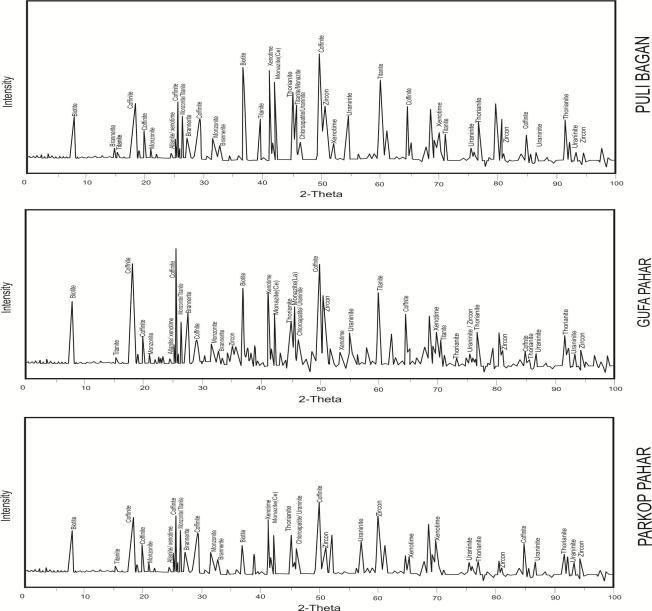
Presentlystudiedgranitesareleucocratic,finetomedium grained, salt pepper textured, essentially composed of quartz,plagioclase,withoccasionalorthoclase,biotites,and microcline. Certain thin sections demonstrate the general indicatorforthepresenceofradioactivenuclideinagranite sampleistheradiohalo(pleochroichalo)showninbiotite andfeldsparmineralscenteredwitheuhedraltosubhedral uraniniteorzirconcrystalsorseenwithemptycentereddue toleachoutofradionuclides(Fig.2a),alphatracksspotted over quartz and feldspar grains (Fig. 2e) and metamict allaniteswithveryfineanastomosingcracksradiatingoutto the adjacent minerals due to emission of alpha radiations (Fig. 2b). The thin sections revealed the presence of accessorymineralssupportedbytheXRDpeaksviz., apatite (1.96), zircon (1.17, 1.80), monazite (1.97, 2.60), xenotime (1.35, 1.83). Primary uranium and thorium phases uraninite(1.60,1.95,1.27), thorianite (1.25, 1.14), brannerite(2.71,3.30),andcoffinite(4.93,3.36,1.84)peaks aresignificantlyreflectedintheXRD(Fig.3).Additionally,Kfeldsparandplagioclasefrequentlyexhibitperthitetexture and with irregular, wormy blebs of quartz in plagioclase developsmyrmekitictexture(Fig.2c),growssimultaneously duringthelatestagesofcrystallizationinthepresenceofa volatile phases. Other hydrothermal alteration textures
Fig-3:RepresentativeXRDpatternsoftheconstituent mineralphasesrepresentingindividualplutonsa)Puli Baganb)GufaPaharc)ParkopPahar
4.2 Geochemistry
The bulk geochemistry of the granite samples is indicatedinthetable(Table1).Thegranitessampleshave high SiO2 wt% that range from 66.76 to 73.95 weight percentages,makingthemacidicrockssupportedbytheir lowMnO(0.06wt%)andTiO2 (0.35wt%)compositions. Al2O3 concentrations range from 13.44 to 14.79 wt%, with14.01 wt% being the norm. Thesamples'average total alkali concentration (Na2O + K2O) is 3.83%, with K2ObeingmoreabundantthanNa2O.ThehighSiO2level of the granite samples indicates that they are substantiallyfractionated.
Uranium concentrations in the examined samples reachupto159ppm,whereas,Thconcentrationsranges (0.37-76.21)ppmandtheTh/Uratioisshowsanaverage of0.59ppm(Table2).The(Fig.4a)granitesamplesdrops below the worldwide border line ratio Th/U (=4) demonstratespostmagmaticallyenrichmentofuranium amid secondary processes (Rogers et al., 1969[9]). The positiveinterdependenceofUandThdemonstratesthe controlofmagmaticprocessesinthedistributionofUand Th (Fawzy, 2017). The uranium mobilisation is represented by an equation U-(Th/3.5). Using the equation, the estimated values for the samples shows greaterthanzerovalue(positivevalue),exceptaParkop Pahar granite (PAKP 3) showing negative value -6.94 indicatinguraniumenrichment.
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 11| Nov 2022 www.irjet.net p-ISSN: 2395-0072
MajorOxide(wt%)concentrationsofNWKarbiHillGranites
Sample GP1 GP2 GP3 GP4 GP5 GP6 GP7 PAKP1 PAKP2 PAKP3 PULI1 PULI2 PULI3 PULI4
SiO2 7395 7251 6676 7364 7374 7326 7379 7079 7085 7146 6968 7022 67.05 7055
Al2 O3 1472 1395 1344 1388 1373 1448 1432 1419 138 1375 1392 139 1333 1479
Fe2O3(t) 1.8 2.44 4.3 1.65 1.71 2.68 2.18 2.94 2.82 2.94 3.23 3.84 4.32 2.3
MnO 0.05 0.06 0.11 0.07 0.06 0.07 0.05 0.05 0.05 0.05 0.06 0.06 0.08 0.06
MgO 0.33 0.39 1.91 0.33 0.25 0.25 0.14 0.34 0.33 0.3 0.48 0.67 1.66 0.41
CaO 1.32 1.21 3.31 1.35 0.88 0.97 0.91 1.14 2.17 2.09 2.03 1.59 3.5 1.36
Na2O 2.72 3.13 2.42 2.17 2.92 2.08 3.37 4.35 3.31 3.06 3.73 3.49 2.47 3.71
K2O 4.25 4.38 4.57 4.99 4.75 4.25 4.42 5.19 5.02 4.34 4.94 4.02 4.14 5.23
P2O5 0.05 0.07 0.65 0.04 0.03 0.03 0.02 0.05 0.06 0.05 0.19 0.15 0.59 0.09
TiO2 0.19 0.31 0.73 0.18 0.19 0.2 0.14 0.23 0.23 0.24 0.56 0.56 0.87 0.32
Table-1: MajorOxide(wt%)concentrationsofNWKarbiHillGranites
Elementalconcentrations(ppm)ofUandThintermsofstudiedgranitesofpartsoffieldareas
SAMPLE GP1 GP2 GP3 GP4 GP5 GP6 GP7 PAKP1 PAKP2 PAKP3 PULI1 PULI2 PULI3 PULI4
U 28.83 22.43 3.95 43.86 55.53 159.10 42.28 20.96 30.72 14.82 3.62 5.75 5.26 16.39 Th 2.72 2.81 0.44 10.02 9.21 54.98 46.69 6.21 4.29 76.21 0.37 1.02 0.50 1.54 Th/U 0.09 0.13 0.11 0.23 0.17 0.35 1.10 0.30 0.14 5.14 0.10 0.18 0.10 0.09 U-(Th/3.5) 28.05 21.62 3.82 3.82 52.89 143.39 28.94 19.19 29.5 -6.95 3.52 5.46 5.11 15.95
Table-2:Elementalconcentrations(ppm)ofUandThintermsofstudiedgranitesofpartsoffieldareas
Amongtherocksamplesthehighestvalue143.39,shown byGufaPahar(GP6).Inthemobilitydiagram(Fig.4c)the rocksamplesshowspositivevalue(3.51-143.39)greater thanzerospecifyinguraniumenrichmentoradditionto therockwhileiftheequationequalstozeroitrepresents absenceofanyuraniummobilisation.Therelationsinthe diagram indicates a lowering trend of Th/U ratio with uraniummobilisationandpostmagmaticredistributionin the studied granites which could lead to a significant economic criterion in the north east A2 type Karbi Hill granites(Boyle,1982[10])disregardingthelonegranite sample,PAKP3.
4.3 Spring Water Characteristics
The results of the geochemical properties (pH, TDS, BIR and LED-fluorimetry) of sampled natural water collectedfromof16(ten)fieldsiteswerecorrelated(Table 3). The attempt for correlative analysis of the chemical parameters portrays the direct relation of TDS ranging from900-6700mg/lvaluewithaquaticBIRrangingfrom 0.02to0.059mR/h.ThehighestTDSmeasuredofsamples waterofParkopPaharalsorecordshighaquaticBIR(0.059 mR/h)atthedischargingsiteofspringwater.Theaverage TDSvalueofNWKarbiHillscalculatedis3130ppm(mg/l). ThehighaquaticBIRaccompaniedwithincreaseTDSofthe natural wateratthefieldsitesmay representsignificant concentration of daughter radio isotopes of U such as radium (226Ra) that is more soluble in poor oxygen environment and fragmentation of uranium bearing minerals(Ahmedet.al., 2019;KittoandKim,2005[11]).
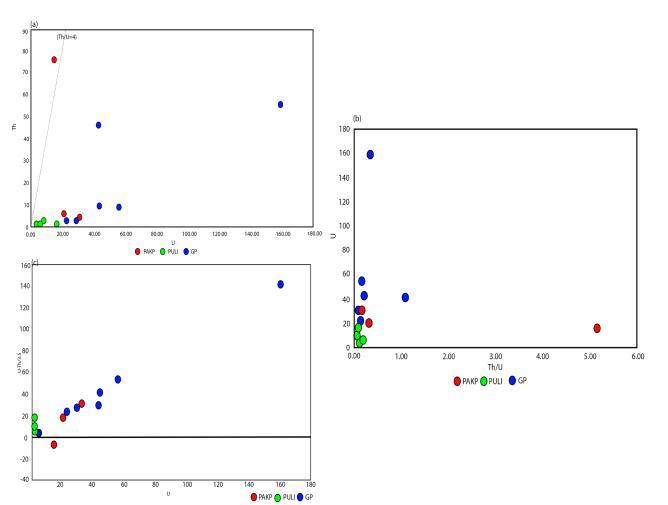
Fig-4:(a)PlottingofNWKarbiHillgranitesontheUversusTh variationplot.(b)TheUversusTh/Uratiovariationplot.(c)The UcontentversusuraniummobilizationequationU-(Th/3.5)plot.
The pH value recorded during the field indicates a significant decreasing value of pH that records considerable amount of background radiation. The measured pH values (5.51-7.83) shows an important relationwithrecordedaquaticBIR.MostofthepHvalues representsslightlyacidictoneutral(5.51-6.38)condition ofnaturalwateraccompaniedbyaconsequentialvalueof BIR. Low pH controls the U mobility, where U4+ gets leached out from the granite rocks and oxidizes to U6+ (Himri et al., 2000). Moreover, the composition of the water,ortheresultofwater-rockinteractionsrelatedto thesolubilityofdifferent rock-forming mineralsandthe kineticsofweathering,alsodeterminesthepHconditionof
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 09 Issue: 11| Nov 2022 www.irjet.net p-ISSN: 2395-0072
naturalwater(Lasagaetal.,1994[12];WhiteandBrantley 1995[13];Whiteetal.2001[14];Bucheret.al.,2017[15]).
Thus,lowpHiscausedbylowCa2+ (calcite)formationor release, low activity acid neutralizing capacity (ANC) chemicals in water, such as HCO3- ions released during cationexchange,orproton-consumingreactionsofprimary mineralssuchasK-feldspar,plagioclase,andbiotite,which results in a fall in the pH condition of natural water sources.Thisisbecausetheresearchareacorrespondsto an acidic rock catchment region. Presence of high concentrationofdissolvedradiumorradon,thedaughter radioisotopes of U decay series in the natural water are favoredbylowpH(Ahmedetal.,2019)andtheseleadsto significant BIR instead of any uranium content (Kumar et.al.,2017[16]).
Statisticalsummaryandcorrelationofgeochemicalproperties pHandTDSwithbackgroundionisingradiationofnatural waterofnorthwestKarbiHills.
LOCATIONS pH TDS(mg/L)/ppm BIR (mR/h)
GPS11 7.52 900 0.016
GPS13 6.9 1000 0.016
GPS14 7.63 1300 0.016
GPS4 7.1 1660 0.017
GPS7 5.7 1700 0.017
GPS1 6.85 2200 0.02
GPS3 7.85 2600 0.02
GPS5 5.76 3000 0.02
GPS6 6.21 3100 0.02
GPS2 6.44 3550 0.021
GPS10 6.21 3600 0.023
GPS12 5.51 4200 0.024
GPS9 6.38 4700 0.028
GPS8 5.5 5100 0.033
GPS15 5.51 5500 0.043
GPS16 5.46 6700 0.059
Table-3: Statisticalsummaryandcorrelationof geochemicalpropertiespHandTDSwithbackground ionisingradiationofnaturalwaterofnorthwestKarbi Hills.
The LED-Fluorimetry analysis of the sampled water (Table?)showinguraniumconcentration<1ppb.InhighpH condition,desorptionofUraniumfromaquifermaterials increaseswiththeformationuraniumcarbonateresulting inhighuraniumconcentrationinwater.While(Choet.al., 2019[17]) reported formation of low soluble U(IV) minerals via reduction of U (VI) in a reducing environmentthatresultsinlowconcentrationofaquatic uraniumconcentration.
5. CONCLUSION
1.XRDisusedtoconfirmtheidentificationofprimaryand secondary uranium phases in thin sections. The radioactivedamagesymptomsseenintheA2typeKarbi granitearetypicalofuraniumenrichedgranites.
2. The slightly acidic to neutral pH conditions of the naturalwaterinthestudyareareflecthighTDSaswellas significantaquaticBIR.Thelowaquaticconcentrationof uranium in the study area indicates a reducing environment due to the reduction of U6+ and the developmentofU4+mineralswithlowsolubility.
4. SignificantaquaticBIRrecordsmayshowanincreasein daughter radionuclide concentrations of the U decay series, such as radium or radon, supported by low pH with increasing solubility due to a low oxidation environmentorreducingconditionandfragmentationof U-Thbearingminerals.
5. Identificationofprimaryandsecondaryuraniumphases in the thin sections are being confirmed by XRD. The symptomsofradioactivedamagefeaturedintheA2type Karbigranitetypifyuraniumenrichedgranites.
ACKNOWLEDGEMENT
The first author is thankful to the Dibrugarh University administration for providing necessary facility for my research work. The necessary laboratory supports were providedbytheDepartmentofAppliedGeologyofDibrugarh University.Theguidanceofunnamedlocalvillagersduring thefieldvisitdeservesspecialappreciation.Logisticsupport provided by the Assam Electricity Board, Missa, Naogaon, Public Works Department (PWD), Morigaon, Naogaon District,Assam(NEIndia)andForestDepartmentofKarbi Anglong during the field study, deserves special appreciations.
REFERENCES
[1 Majumdar D, Dutta P. Geodynamic evolution of a PanAfricangranitoidofextendedDizoValleyinKarbiHills,NE India: evidence from geochemistry and isotope geology. JournalofAsianEarthSciences.2016Mar1;117:256-68.
[2]KakatiRK,BhattacharjeeB.EstimationofAlphaActivity in Various Sources of Water in Different Places of Karbi Anglong District of Assam, India. International Journal of PureAppliedPhysics.2011;7(1):1-5.
[3] Ahmad N, ur Rehman J, Rehman J, Nasar G. Effect of geochemicalproperties(pH,conductivity,TDS)onnatural radioactivityanddoseestimationinwatersamplesinKulim, Malaysia. Human and Ecological Risk Assessment: An InternationalJournal.2019Oct3;25(7):1688-96.
2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page402

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 09 Issue: 11| Nov 2022 www.irjet.net p-ISSN: 2395-0072
[4] Kozłowska B, Dorda J, Kłos B, Przylibski TA. Radium isotopes on bottled mineral waters of Outer Carpatians. Poland.InProceedingsofthe2ndInternationalConference on Radioactivity in the Environment, France. Strand, P., Borretzen,P.andJolle,T.,Eds2005.
[5]HimriME,PastorA,dela GuardiaM.Determination of uranium in tap water by ICP-MS. Fresenius' journal of analyticalchemistry.2000May;367(2):151-6.
[6]RobinsNS.GroundwaterqualityinScotland:majorion chemistry of the key groundwater bodies. Science of the TotalEnvironment.2002Jul22;294(1-3):41-56.
[7] Murlidharan PK, Raj D. Systematic geological mapping anddetailedinvestigationaroundultra-basicoccurrencesin thecentralandnorthernpartsof‘KarbiAnglong’(MikirHills) District.Assam.Rec.Geol.Surv.India.1983;111.
[8]BoraAK,RoyG.Preliminaryinvestigationforgoldand othermineralpotentialitiesintheareaaroundKaliyaniand Diju Valley, Karbi Anglong District, Assam. Rec. Geol. Soc. India.1999;130:16
[9]RogersJJ.Uranium.Handbookofgeochemistry.1969.
[10] Boyle RW. Geochemical prospecting for thorium and uraniumdeposits.Elsevier;2013Nov11.
[11]KittoME,KimMS.Naturallyoccurringradionuclidesin community water supplies of New York State. Health Physics.2005Mar1;88(3):253-60.
[12] Lasaga AC, Soler JM, Ganor J, Burch TE, Nagy KL. Chemical weathering rate laws and global geochemical cycles. Geochimica et Cosmochimica Acta. 1994 May 1;58(10):2361-86.
[13] White AF. " Chemical Weathering Rates of Silicate Minerals.Reviewsinmineralogy.1995;31.
[14] White AF, Brantley SL. The effect of time on the weathering of silicate minerals: why do weathering rates differinthelaboratory and field?Chemical Geology.2003 Dec30;202(3-4):479-506.
[15]BucherK,ZhouW,StoberI.Rockscontrolthechemical compositionofsurfacewaterfromthehighAlpineZermatt area (Swiss Alps). Swiss Journal of Geosciences. 2017 Oct;110(3):811-31.
[16]KumarMR,RajuPS,DeviEU,SaulJ,RameshDS.Crustal structure variations in northeast India from converted phases.GeophysicalResearchLetters.2004Sep;31(17).
[17]ChoYJ,KimIH,ChoYJ.Numericalanalysisofthegrand circulation process of Mang-Bang beach-centered on the
shorelinechangefrom2017.4.26to2018.4.20.Journalof Korean Society of Coastal and Ocean Engineers. 2019;31(3):101-14.
[18]EvansP.TectonicframeworkofAssam.J.Geophys.Soc. India.1964;5:80-96.
[19]FawzyKM.CharacterizationofapostorogenicA-type granite, Gabal El Atawi, Central Eastern Desert, Egypt: geochemicalandradioactiveperspectives.OpenJournalof Geology.2017;7(01):93.
[20]GulanL,SpasovićL.Outdoorandindoorambientdose equivalent rates in Berane town, Montenegro. InRAD5 Proceeding of Fifth International Conference on radiation and applications in various fields of research. RAD Association.doi2017(Vol.10).
[21] Meert JG. What's in a name? The Columbia (Paleopangaea/Nuna)supercontinent.GondwanaResearch. 2012May1;21(4):987-93.
[22] Rogers JJ, Santosh M. Configuration of Columbia, a Mesoproterozoicsupercontinent.GondwanaResearch.2002 Jan1;5(1):5-22.
[23]RogersJJ,SantoshM.Continentsandsupercontinents. OxfordUniversityPress;2004Sep16.
[24]SrivastavaSK,HamiltonS,NayakS,PandeyUK,Mohanty R,UmamaheswarK.Petrography,geochemistryandRb-Sr geochronologyofthebasementgranitoidsfromUmthongkut area,WestKhasiHillsdistrict,Meghalaya,India:Implications onpetrogenesisanduraniummineralization.Journalofthe GeologicalSocietyofIndia.2015Jul;86(1):59-70.
[25] Wegener, A. "The Origin of Continents and Oceans. (Engl.Trans.)London."(1924).
2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal |

