International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072
Department of Electronics and Instrumentation, School of Physical Science, Bharathiar University, Coimbatore-46, India***
ABSTRACT - The aim of the research is used to demonstrate a Multi-walled Carbon nanotube (MWCNT) based biosensor to identify the selective determination of bacteria like Staphylococcus aureus. It is based on the (FET) field-effect transistor in which a linkage of multiwalled carbon nanotube acts as a conductor channel. Staphylococcus aureus antibodies are analyzed by the indirect ELISA method. CNT sensor is immobilized on pAbs after the hybridization of (pBASE) 1-pyrenebutanoic succinimidyl ester. The resistance variation can be measured by a potentiostat. Our proposed transistor was uncovered to develop a high-level concentration of the staphylococcus at least 100 CFU/ml. It was absorbed by the Scanning electron microscope (SEM). The sensitivity and selectivity of the transistor can be analyzed. The concentric value of MWCNT can be determined to be 0.1 mg/mL. This device is used to detect bacteria by suitable antibodies, bacteria, and viruses. The resulting interaction between pAbs with Staphylococcus aureus is increased significantly in the resistance value of the biosensor (P<0.07). The MWCNT-based biosensor can detect Staphylococcus aureus with a detection limit is 2log CFU/mL.
Key Words: Staphylococcus aureus, Multi-wall carbon nanotubes, Field effect transistor, Antibodies, pBASE.
Nanotechnology is a field of nature that belongs to all features of human life [1]. A Carbon nanotube has its allotropy property with a structure of cylindrical nanotubes that classifies as single-walled carbon nanotubes(SWCNT)andMulti-walledcarbonnanotubes (MWCNT). The types could be functionalized or nonfunctionalized due to the more cohesion for the diverse substrate [2]. The properties of MWCNT are large surface area, thermal conductivity, or tensile strength is used for the applications of thermal and chemical processes bypost-synthesis techniques [3].Annealing is the process to reduce structural defects and remove the catalytic metals that are used in the synthesis process. The dispersity is increased in an aqueous solution and improves the compatibility with composite materials due to the functionalization with strong oxidizing [4].
The infection of microbial causes serious risks to humans with different types of diseases that may cause death [5]. CNT plays a significant role in the growth control of microbial so the CNT will apply in different applications like tissue engineering, biosensing application,drugdelivery,anddressingthewoundandit helps to enhance the strong antimicrobial activity [6].1PyrenebutyricacidN-hydroxysuccinimideester(PBASE) covalent bonding with CNT helps the antimicrobial activitiesofStaphaureus.CNTcontactdirectlywillcause cell damage and cell death [7, 8]. The size and surface area of the CNT are the main parameters that affect the antibacterial activity to interact with the bacteria [9]. The enzyme immunoassays merge the antigen-antibody reaction with the signal amplification and sensitivity for the catalyzed materials and the conventional electrochemical methods that can be operated in the crude samples without the filtration or separation methods.Thenon-specificbindingofbacteriaorprotein can be avoided by the PBASE. Staph aureus is detected by the standard plate count method with the selective enrichment in the broth prepared. The resistance of the organism increased towards the antibiotic which can be led to severe health problems and cause bacterial infection was resistant to the antibodies to remove the infection [10]. The MWCNT-based sensor is synthesized based on the field-effect transistor that nanotubes are used as an electron channel between the sources and drain electrodes. MWCNT-FET biosensor is applied for the detection of aureus due to its ability to detect the interface from absorption of charged species [11]. The MWCNT will be connected to an electrochemical electrodebasedonasurfacemodificationtoimprovethe electron transfer rate and surface area [12]. The interaction of antigen-antibody connected with the sensinglayeroftheCNTtotransducethechargetransfer oftheFETcanbeusedtodetectthebacteria.Thegoalof ourresearchistoevaluatethesensorandcharacteristics of the devices to detect Staphylococcus aureus. The objectives of the research are to analyze the antibodies present in staph aureus for biosensors, develop a fabrication step for MWCNT-based biosensors, and also characterize the properties of CNT-based sensors to detectstaphaureus.
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072
DMF, TFA, and PBASE were purchased from (LobaChemic).Thecultureofthestaphylococcusaureus was purchased from Sigma-Aldrich Solutions. pAb was obtained from Thermo fisher scientific and diluted to a concentration of 4 mg/ml with carbonate-bicarbonate buffer. The other reagents are used in the analytical grade.
MWCNTissynthesizedbyaCVDmethodandthe ironnanoparticlesareusedasthecatalyst.Thenanotube wasmadeonasiliconsubstratethatcontainedacatalyst in the thin film and the two buffer layers with 2 nm of iron, 15 nm of aluminum oxide, and silicon dioxide. Ethyleneisthesourceofcarbon.Nanotubewillbegrown in the oven at a temperature of 700-800℃. The silicon substrates contain the catalyst that was placed on the feeder which was in the presence of H2 and Ar in the vacuum oven and the substrate is heated for up to 15 minutes. At the time of heating, hydrogen gas was put into the chamber and it is supplied for 45 minutes. For the growth process, the supply of hydrocarbon gases is stopped and the feeder is taken out of the oven. The systemwasremovedwiththepresenceofArpoured for 5minutesandtheovencooledslowlyto200℃[13].The process was completed by cleaning the whole system. The 3 mg of MWCNT powder was dissolved in 18 ml of DMF and 2 ml of TFA was sonicated for 1 hour. The mixture was centrifuged at 15,000 rpm for 30 minutes. The solvent was poured and the settled CNT dissolved againinthesameprocess.Finally,thecarbonnanotubes are diluted with toluene and the solution occurs colorless and transparent. The dispersion was analyzed bytheSEM.
The MWCNT is functionalized by modified Hummer’s method. The 2 g of MWCNT is treated with the 1 g of NaNO3 solution was mixed with 50 ml of Concentrated H2SO4 in a 250ml conical flask. The solution was stirred for 1 hour in an ice bath and followed by 3 g of KMnO4 was append to the solution under vigorous conditions. The rate of addition is controlled carefully to keep the reaction temperature below 20℃. After removing the reaction mixture from theicebathandthesolutionwasstirredfor1hrat35℃ Then 46 ml of distilled water is added slowly and the mixture was stirred for 30 mins. Finally, 15 ml of hydrogen peroxide and 300 ml of Distilled water were added slowly to the suspension. The solution was centrifuged with 10% hydrochloric acid and distilled
water several times. The washing process will be repeateduntilthepHisneutral.Thesolutionwaskeptin the hot oven at 60℃ for 24 hrs to obtain the functionalizedMWCNTasagrey[14].
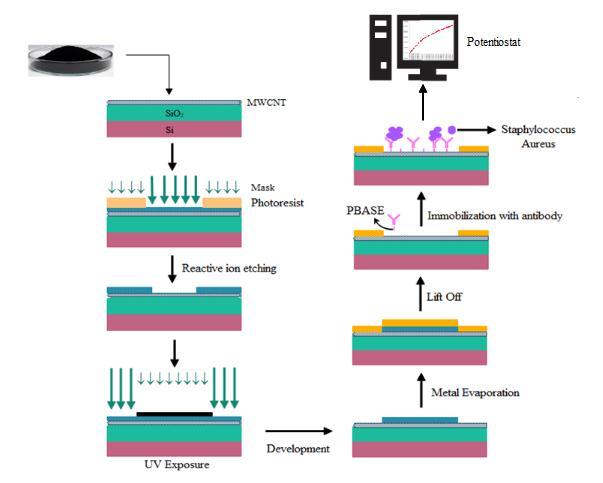
The Dielectrophoresis method is used for thermally oxidized Si wafers with electrode pairs consisting of metallic electrodes like gold (Au). The electrodes are patterned by photolithography and oxidized with silicon wafers. The n-type silicon wafer is usedfordryoxidationatatemperatureofabout1100℃. The wafer was covered on both sides with 100 nm of silicon dioxide. The backside was etched by buffered oxide solution (BOE) with a 500nm aluminum thin film deposited on the backside gate contact using e-beam evaporation. The electrodes are prepared by e-beam lithography 50 µm long and 500 nm wide with a gap of 800 nm between the electrodes. The 100 nm gold thin films are developed by e-beam evaporation. For the dielectrophoresisposition,a6µldropofMWNTsolution wasdispersedonthedevice.Thesolventwasappliedon thesurfacewithnitrogengasafter2min,andthedevices were annealed at 300℃. Figure 1 shows the overall processforthefabricationofbiosensors.
The culture was prepared in a test tube by inoculating BHI broth with the bacteria moved from the plate to the test tube by a cotton wipe. Staphylococcus aureus was kept in broth by incubating for 16 hrs at 37℃. The culture was washed 5 times with 15 mM PBS bycentrifugingat6000rpmfor10minat4℃.pAbswere dilutedwithaconcentrationof4mg/mLwithcarbonate bicarbonatebuffer.
Thebacteria werecollectedandwashedseveral timesat15mMPBSbuffer bycentrifugingat6,500rpm for 15 mins at 5℃. A chemical reaction of 100µL antiStaphaureus antibodieswasvariedwithPBSbufferand incubated with the microplate. The growth of color change is measured using a microplate reader and the resultwascalculatedusingthebelowequation(1)[15].
Differenceof =Resultedvalue–ResultedValue
Absorbance after30min after0min (1)
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072
The concentration of PBASE is used as a linker on the biosensor for the Sensing event that affects the biosensor signal. The fabricated MWCNT-FET biosensor was tested with the active amount of linker with the concentration known for 2 hours at normal room temperature. The resistance is used to analyze the identity of the optimal linker concentration at the connection between the receptor and the MWCNT fieldeffecttransistor.
The non-covalent functionalization of the MWCNTisusedtoconfigurethebiosensorwithaPBASE linker (6 mM) as a linker for 2 hours at room temperature. It is followed by the cleaning of distilled water to remove the unwanted PBASE on the biosensor [16].4mg/mlofantibodywascentrifugedat15,000rpm for 10 mins and diluted with buffer solution. The antibodywasattachedtothesurfacesoftheMWCNTby showingtheantibodytothelinkageofPBASEwithbeing settled overnight at 4℃, to allow the formation of
covalent bonds. The MWCNT-based biosensor is attached to the pAbs to wash with PBS buffer (pH 7.4). Thepotentiostatisusedtomeasuretheresistancevalue oftheantibodysurface.
The Staphylococcus aureus cells are collected fromthefull-growncultureafter3timesofwashingwith 10 mM PBS (pH 7.4). The solution was centrifuged at 5000 rpm for 20 mins at 5℃. The bacteria were sensed by the optical density (OD) which is measured by 650 nmusingaspectrophotometerwithastandardcurvefor log CFU/mL. 0-1 mL of aureus culture solution was dispersed on the MWCNT-based biosensor for 30 mins andincubatedatroomtemperaturetoallowthebonding connection between the bacteria and antibody. The sensor was prepared and spread over the PBS buffer on the biosensor that was attached to the antibodies. The potentiostat is used to analyze the linear sweep voltammetry curves after the incubation. The current and voltage curves are calculated between the linear analyses with the resistance (R) can be measured by
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072
inversingthevalues[17].Theresistancedifference(��) canbecalculatedusingthebelowequations: ��=(R1-R0)/R0 (2)
WhereR0isthelinkerresistancevalue, R1 isStaphylococcusaureuscultureresistancevalue.
The indirect ELISA method is implemented to showthedetailedbindingoftheStaphaureuswithpAbs. This method consists of primary antibodies connected with the enzymes and secondary antibodies reacting with the primary antibodies that reacted with an enzyme. While the enzyme is reacted with substrates that can produce a fluorescent compound, changes in a color reaction change between the enzymes and substrate that are connected with the secondary antibodies [18]. The concentration of the bacterial species is 1 X 108 CFU/mL. When the pAbs are distilled severaltimesandtestedonthebiosensor,theresistance ofthebiosensorincreasesgradually.ThisindirectELISA method is only suitable for the specific binding of pAbs to aureus and also the antibody is used to develop the biosensortodetectthestaphaureus.
The antibody into the MWCNT involves a connectionbetweentheCNTsurfaceandantibodies.The species can be adsorbed by noncovalent on the MWCNT surface through ℼ-ℼ stacking, hydrophobic, and electrostatic interaction. The linker of the CNT surfaces is pyrenebutanoic acid, and succinimidyl ester [19]. The PBASE of the hydrophobic pyrenyl group will adsorb irreversibly into the sidewall of the CNT through a ℼ-ℼ stacking interaction. Figure 2 shows the binding of PBASEwiththeMWCNTsurfaceandtheresistancevalue willnotchangethesensor.Thesuccinimidylesterwillbe groupedbyanotherendofthe1-pyrenebutanoicreacted withthesecondaryandprimaryaminesonthesurfaceof the antibodies for nucleophilic substitution in the occurrence of the DMF solvent. Immobilization is achieved by the amino group of the antibody with the linkertoformcovalentamidebonds.
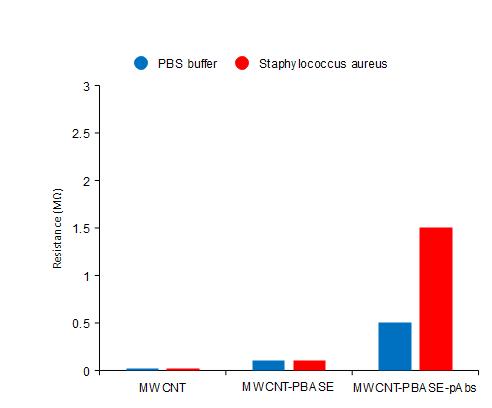
Microbial metabolism generally resulted in an increasing type of conductance and capacitance because ofreducedimpedance.Inouranalysis,the bacteria bind with antibodies to increase their resistance and can be monitored by the potentiostats. The resulting current flow is increased in the resistance value due to the interaction between the antibody and microorganism [20]. Figure 3 shows the detection values of the sensor with Staph aureus analyzing the fabrication of the individual steps of the biosensor. There is no difference in the resistance values between the buffer solution of PBS and bacterial culture. The biosensor is immobilized withtheMWCNT,MWCNTwithPBASE,andpAbs.There is a major difference in resistance between the PBS and bacteria after the sensor immobilization with antibody (P < 0.07). The resistance will be increased from the specificbindingbetweentheantibodyandStaphaureus.
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072
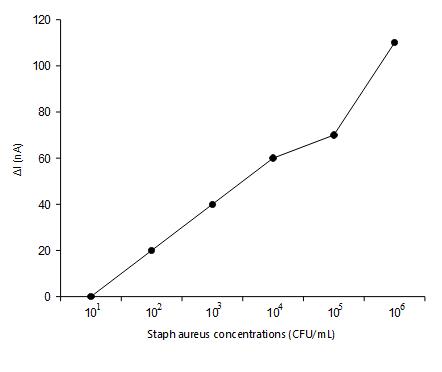
Sensitivity analysis was shown to define the biosensors with a limit of detection. A Series of 10-fold serial dilutions was prepared from the stock culture using0.1%ofpeptonewater[21]. Theserialdilutionsof Staph aureus were analyzed with the sensor and the concentration of Staph aureus cultures from 101 to 108 CFU/mL was shown in figure 4. The measurement of electrical current showed a linear characteristic among the current (ΔI) and concentration of the aureus suspensionwithintherange of108 CFU.Thecellculture is increased and the current becomes decreases. While thecurrentisincreased,theincreasedbacterialisloaded to the MWCNT-FET surface. The occurrence of antibodies will react with the junction to become saturatedwiththeconcentrationoftheantigenwiththe high changes in the electrical properties of the MWCNT. The detection limit of the proposed sensor is 102 CFU/ mLwithlessdetectiontimeof5mins.
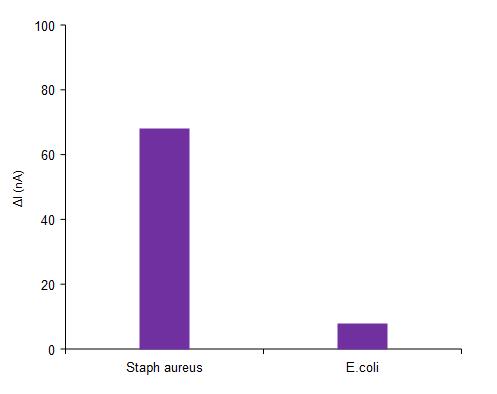
-5: SpecificityofthebiosensortowardStaphaureus
The chemical composition and structure of the MWCNTwerecomparedwiththeironcatalystandfigure 6showstheXRDpatternofthepeakvalue. Thesamples showed the characteristics Bragg diffraction peak of the MWCNTs at 2θ = 26º. The peak value displays the presenceofcarbonandalsothestructureofthepowder was analyzed as hexagonal regarding the JCPDS database. Theintensityofthepeakisdecreasedwiththe increasing loading rate of the iron ratio for MWCNTs. The XRD result shows the data about the lattice that is primitive and crystalline. The size of the crystalline is determinedbytheScherrerequation[22],
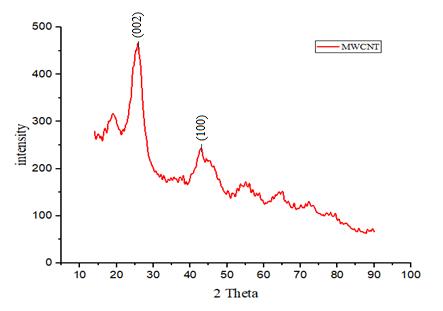
Fig -4: Relationshipbetweenthecurrentchangesand concentrationofStaphaureus
Specificity testing is used to measure the ability of the sensor for the target species. The target analysis sensor was located inside the surface to evaluate the sensortodetecttheStaphaureus.TheE.coliwasapplied to the sensor, the value can be measured using the current was 7.29 nA, which can be assigned to the nonspecific binding of E.coli on the surface. Figure 5 shows the relative response of the biosensor to the Staph aureus. The highest value of 66.77 nA is measured with staph aureus which shows the functionalization of the MWCNT-FET biosensor. Sterile 0.1% peptone water is used as the negative control. The specificity was carried out by the biosensor towards the concentration of bacteriawithE.coliandStaphaureus.
Dp=Kλ (3) βcosθ
Where,Dp =AverageCrystallitesize,β=Linebroadening inRadius,θ=Braggangle,λ=X-raywavelength.
Fig -6: XRDpatternoftheMWCNT
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072
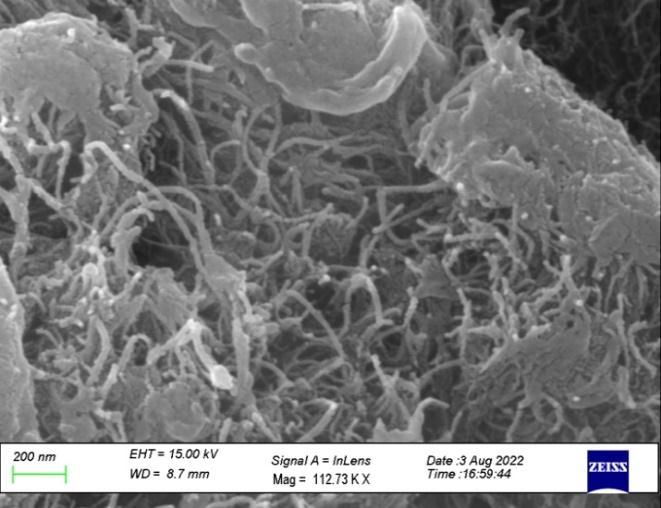
The MWCNT is synthesized from the Chemical vapordepositionmethodandisanalyzedforitssizeand morphological structure using a scanning electron microscope. The carbon nanotubes are grown on the silicon substrate with the iron catalyst. The images attainedfromtheFESEManalysisthatfigure7showsthe size and type of growth of the CNT. The diameter of the synthesized carbon nanotube was produced (approximately 10-15 nm). The specimen is placed directly in the position in the fabrication process. The growth of the carbon nanotube occurs from the iron catalyst due to high temperatures. It confirms the number of bacteria attached to the CNT was proportional to the concentration of Staphylococcus aureus.
The biosensor acted with PBS buffer that exhibited in a typical design tangled-shaped MWCNT on the surface. Figure 9 shows the SEM image exposed the aureuscaughttheexternaloftheCNT-basedbiosensor.
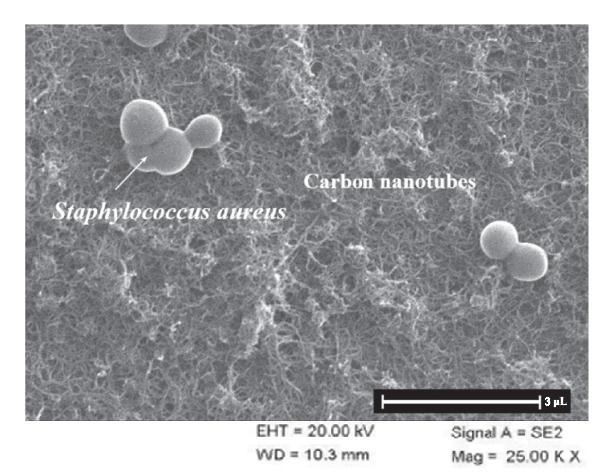
Fig -7: FESEManalysisoftheMWCNT
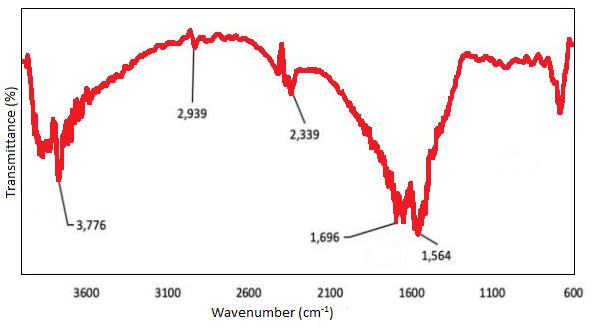
FT-IR is mostly used as the qualitative method for the evaluation of functional groups. Figure 8 shows the spectra of the MWCNT from 600 to 3600cm-1. It shows the carbon stability and the hexagonal structure from the MWCNT peak at 1564-1600cm-1 illuminating thecarbondoublebonding(C=C).Thepeakat3776cm-1 is determining the –OH stretching of MWCNT. The C=C showstheoxidationofcarbonwitharemarkablepeakof 1696cm-1 asthecarbonylofthecarboxylgroup.Theacid solution attacks the carbon double bonding at the decreasing peak at 1564 cm-1 and hexagonal carbon at the region 600-1300 cm-1. Functional groups analyzed ontheCNTsurfacewerevaluedbytitration.
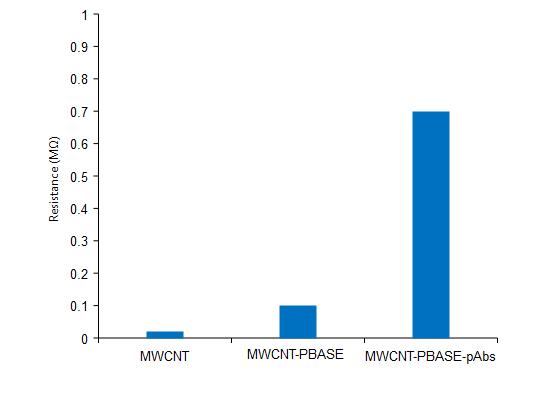
TheSEMimagedisplaysabiosensoronMWCNT that will detect the aureus cells that resulted in the resistancevaluesobtainedintable1.Thebiosensorwas attached with PBASE only because it cannot able to capture the cells which exhibited the resistance differencesbetweenthePBSbufferandbacterialculture. Table 1 shows the difference between the antibody attached to the PBASE and the resistance in the biosensor (P < 0.07). The resistance will increase after the functioning of the biosensor with the antibody for Staphaureusandtheresistancewillincreasebecauseof the binding of the antibody with the bacteria on the SWCNTbytheGarcia-Aljaro[17].
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072
ImmobilizationStep Resistancedifference(��)
PBASE 0.03± 0.08
PBASEandpAbs 9.76± 0.48
Table -1: Effectofimmobilizationstepontheresistance differenceonMWCNT-basedbiosensor
This work displays that the MWCNT-based biosensor could be suitable for the recognition of foodborneinfectionssuchasS.aureuswithafield-effect transistoronMWCNT.TheindirectELISAmethodshows the anti-S. aureus polyclonal antibodies will target the cells.Thegoldelectrodesfabricatedonthesiliconwafer were accumulated with multi-walled carbon nanotubes. The assembled MWCNT was determined to be the 1pyrenebutanoicacid,succinimidyl esterwaschosenasa linker to bind between the MWCNT and pAbs. The PBASEandpAbswereimmobilizedonthetransistorand the resistance of the MWCNT will be increased. The captured Staph aureus on the MWCNT will be displayed usinganSEM.Ifthenumberofbacteriaisincreasedthen the resistance decreased. The MWCNT-based biosensor was developed to analyze the Staphylococcus aureus withalowconcentrationof102 CFU/mL. Therefore,the maximumdetectionofthebiosensorwasanalyzedtobe 2logCFU/mL.
This research was supported by the promotion ofUniversityResearchandScientificExcellence(PURSE) -phaseII,DepartmentofScienceandTechnology(DST), Bharathiar University, Coimbatore, Tamilnadu. Award LetterNo.:BU/DSTPURSE(II)/APPOINTMENT/597.
[1]. Liu,Jian,etal.Small7.4(2011):425-443.
[2]. Jung, Dae-Hwan, et al. Materials Science and EngineeringC24(2004):117-121.
[3]. Balasubramanian, Kannan, et al. Small 1.2 (2005): 180-192.
[4]. Xing, Yangchun, et al. Langmuir 21.9 (2005): 41854190.
[5]. Sethi, Sanjay, et al. Clinical microbiology reviews 14.2(2001):336-363.
[6]. Kang, Seoktae, et al. Langmuir 23.17 (2007): 86708673.
[7]. Amiri, Ahmad, et al. Materials Letters 72 (2012): 153-156.
[8]. Kang, Seoktae, et al. Environmental science & technology42.19(2008):7528-7534.
[9]. Kang, Seoktae, et al. Langmuir 24.13 (2008): 64096413.
[10]. Rippers, R.A. edited by V. Lorian, Williams and Wilkins:Baltimore,(1980).
[11]. Villamizar, Raquel A., et al. Biosensors and Bioelectronics24.2(2008):279-283.
[12]. Zhao, Guangying, et al. Analytical biochemistry 408.1(2011):53-58.
[13]. Dobrzańska - Danikiewicz, A.D., et al. Methods 2 (2014):3.
[14]. Karthika,Viswanathan,etal.IETNanobiotechnology 11.1(2017):113-118.
[15]. Zhang, Shuqing, et al. Journal of food science 71.3 (2006):M100-M104.
[16]. Lee, Jin Young, et al. Biosensors and Bioelectronics 26.5(2011):2685-2688.
[17]. Garcia-Aljaro, Cristina, et al. Biosensors and Bioelectronics26.4(2010):1437-1441.
[18]. Park, Mi-Kyung, et al. Sensors, and Actuators B: Chemical171(2012):323-331.
[19]. Ivnitski, Dmitri, et al. Biosensors and bioelectronics 14.7(1999):599-624.
[20]. He, Pingang, and Liming Dai. In BioMEMS and biomedical nanotechnology, pp. 171-201. Springer, Boston,MA,2006.
[21]. Yamada,Kara,etal.PLoSOne9.9(2014):e105767.
[22]. Arunkumar, T., et al. International Journal of AmbientEnergy41.4(2020):452-456