International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072
1Assistant Professor, Sherwood College of Pharmacy Barabanki, U.P, India.
2Associate Professor, Ramswaroop College of Pharmacy Lucknow, U.P, India.
3Lecturer, Sherwood College of Pharmacy Barabanki, U.P, India.
4 Lecturer, Sherwood College of Pharmacy Barabanki, U.P, India.
5 Assistant Professor, Sherwood College of Pharmacy Barabanki, U.P, India.
***
Abstract - Plants have a wealthofmedicinalchemicalswith vast potential in the pharmaceutical sector. The purpose of this research was to determine which phytochemicals were present in the seven medicinal plants chosen for this study, as well as to determine whether or not these plants have any antibacterial or antioxidant properties. Methods. The total phenolic and flavonoid contents, as well as the results of a standard phytochemical screen, were calculated. The antioxidant activity of plant extracts was determined using 2, 2-diphenyl-1-picrylhydrazyl (DPPH),hydroxyl(OH),andnitric oxide (NO) radical scavenging assays.Thebrothmicrodilution technique was used to test the plant extracts for antibacterial activity. Phenols, flavonoids, and steroids were found in every plant extract analyzed by phytochemicalmethods.Theextract of Psychotria peduncularis showed the highest total phenolic and flavonoid contents (5.57 •} 0.22 mg GAE/g and 1.38 •} 0.06 mg QE/g, respectively). The IC50 values for DPPH radical scavenging and NO radical scavenging ranged from 0.55 to 49.43 g/mL and 0.65 to 13.7 g/mL, respectively, indicating that all plant extracts exhibited extremely significant antioxidant activity. With MIC values rangingfrom16to1024 g/mL, extracts of both Tristemma Mauritian rum and P. peduncularis showed potent antibacterial activity. Extractsof T. Mauritian rum were bactericidal against all species examined. There was substantial antifungal activity (MIC 64 g/mL) observed between Candida albicans and extracts of Alsophila Marianna and P. peduncular. Our research suggests that the screened extracts of medicinal plants might be employed as resources for the creation of novel medications, namely as antioxidant and antibacterial agents.
Key Words: Microbial Pathogens, Medicinal Plants, Antioxidant,Antimicrobial,DPPH
Antimicrobial resistance is a growing problem due to the creation and spread of drug-resistant bacteria that have developednovelresistancemechanisms[1].Multi-andpanresistantbacteria(alsoknownas"superbugs")arerapidly spreading overthe world,causingdiseasesthatcannot be
treated with conventional antimicrobial medications like antibiotics or antifungals [2]. There are no new antimicrobials under clinical development. 32 medicines targeting WHO priority diseases are now under clinical research,althoughonlysixofthemmaybeconsiderednovel. Moreover, a significant problem is still the limited availability of effective antimicrobials. Shortages of antibiotics and antifungals have a significant impact on healthcare systems worldwide, regardless of economic growth [3, 4]. Overproduction of reactive oxygen species (ROS)hasalsobeenlinkedtotheonsetofa widerangeof chronicanddegenerativeconditions,includingcancer,and respiratory,neurological,andgastrointestinalillnesses[4]. Antioxidants, which may be produced endogenously or suppliedexogenously,haveafinelytunedroleincontrolling ROSconcentrationsunderphysiologicalsettings.
Antioxidant deficiency, in conjunction with starvation,maymakepeoplemoresusceptibletooxidative stress, which in turn raises the probability of cancer incidence[4].Furthermore,persistentinflammation,asseen inCOPD,IBD,neurologicalillnesses,cardiovasculardisease, and even aging [5], may cause the antioxidant defense to become overwhelmed. Vitamin D, an antioxidant vitamin, playsacrucialroleincontrollingbiochemicalprocessesthat ensurehealthyorganfunction.Someclinicalevidenceshows thatantioxidantsupplementationmayreduceendogenous antioxidantdepletionandtheresultingoxidativedamage[6]. Since antibiotic-resistant microorganisms are on the rise, andsincereactiveoxygenspecies(ROS)areimplicatedina wide range of chronic and degenerative human diseases, scientistshavebegunlookingforplant-basedantioxidants andbioactivecompoundswithnovelmechanismsofaction to combat pathogenic microbes [7, 8]. To make informed decisionsontheusageofmedicinal plants,onemusthave access to reliable scientific data and a familiarity with the plants' chemical constituents. Plants have medicinal propertiesowingtochemicalmoleculesinsidethem[9].The use of medicinal plants is crucial in the search for safer alternativestomanufacturedpharmaceuticals[10,11].Both conventionalandalternativemedicinehavelongreliedon
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072
plants and other natural ingredients, which are now commonly employed in the industrial medication manufacturing process. According to credible scientific studies [12, 13], herbs account for almost 25% of all pharmaceuticalsusedaroundtheglobe.
Although these plants have long been used for therapeuticpurposes,notmuchresearchhasfocusedonthe phytochemicalcomponentsoftheseplants.Furthermore,the antioxidant and antibacterial effects of these therapeutic herbs are little researched. Therefore, the purpose of this research was to assess the antioxidant and antibacterial activityofextractsfromthesetherapeuticplants,aswellas theirphytochemicalcontents.
Fourfungalstrains: Candida albicans (ATCC90029), Candida parapsilosis (ATCC 22019), Candida krusei (ATCC 6258), and Candida tropicalis (ATCC 750) were used. The bacterial spp. used were Escherichia coli (ATCC 10536), Staphylococcus aureus (ATCC 25923), and Enterobacter atherogenesis (ATCC 13048), and three clinical isolates, namely, ProvidenciaStuart, P.aeruginosa, and Vibriocholerae C06. The ATCC was a source for the fungi and bacteria, whereasthePasteurInstituteYaound'ewasthesourcefor the clinical bacterium isolates (Cameroon). The bacterial activationandantimicrobialtestsbothmadeuseofMueller Hintonagar(MHA,DominiqueDeutscherSAS)andMueller Hintonbroth(MHB,DominiqueDutscherSAS).Sabouraud Dextrose agar (SDA, Liofilchem) and Sabouraud Dextrose broth(SDB,Liofilchem)wasusedfortheactivationofyeasts andantimicrobialassays,respectively.
Seven fresh plant specimens, including H. decumbens,L.macrocarpa,T.mauritianum,C.stelluliferum, A. manianna, C. bougheyanum, and P. peduncularis, were collectedinSeptemberof2016fromavarietyoflocations within the Tombel subdivision, which is situated in the southwesternregionofCameroon.AtCameroon'sNational Herbarium,theplants'provenanceasnativetothecountry was examined and confirmed. The voucher numbers that were allotted to the various plants are listed in Table 1, whichmaybeseenhere.
Afterbeingharvested,theplantswerewashedwith water,andafterthat,theywerelaidoutintheshadeandleft to dry at room temperature. After the plant samples had been dried, they were ground into powder, and then one hundredgramsofthatpowderfromeachplantsamplewas macerated in eight hundred milliliters of methanol. After
that, each sample was filtered using Whatman No. 1 filter paper,andthemethanolwasextractedfromeachfiltrateby runningitthrougharotaryevaporator(BuchiR-200)while the pressure was decreased. Finally, the samples were combinedandanalyzed.Theextractswerekeptfrozenata temperature of 4 degrees Celsius in anticipation of future studies.
2.4.Preliminary
Wewereabletodeterminewhetherornotcertain chemicals were included in each plant extract by using a method that had been devised by Harbone (1984) [28]. These components comprised, amongst others, alkaloids, steroids, glycosides, flavonoids, tannins, saponins, and terpenoids. Both the total phenolic content (TPC) and the total flavonoidcontentwere determinedwiththeuseof a techniquethatwasestablishedbyDzoyemandEloff[29].
3.1.
Dzoyem and Eloff's[29]protocol wasfollowed to conduct the DPPH test. Simply put, 100 L of each plant extract sample ranging in concentration from 12.5 to 200 g/mLwascombinedwith900LofDPPHsolution(0.2mM) produced in methanol. After 30 minutes of incubation at roomtemperatureandinthedark,thespectrophotometer readingwastakenat517nmtodeterminetheconcentration of the combination. Positive control was performed using ascorbic acid, a negative control was performed using methanol,andablankwasperformedusingextractwithout DPPH. Formula: %I = ((AbsorbanceControl AbsorbanceSample)/AbsorbanceControl)100indicatesthe percentage of inhibition of DPPH radical scavenging. By graphingpercentagesofinhibitionagainstconcentrationsof eachsample,theIC50 requiredtoscavenge50%ofradicals fromeachplantextractwasdetermined.
Each plant extract's ability to scavenge hydroxyl radicals was measured using a modified version of the FentonprocesspublishedbySowndhararajanandKang[30]. To recap, 1.5 mL of each plant extract at varying concentrations(12.5-200g/mL)wascombinedwith90Lof FeCl3(4mM)and60Lof1,10-phenanthrolineinavolume of1.5mL.(1mM).Then,150LofH2O2(0.17M)and2.4mL ofphosphatebuffersaline(0.2MpH7.4)wereadded.After 10 minutes of standing at room temperature, the spectrophotometer was used to measure the reaction mixture'sabsorbanceat560nmtodeterminetheoutcome of the reaction. A buffer solution served as a negative control,whereasascorbicacidwasemployedasapositive one.AswiththeDPPHradical scavenging experiment,the
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072
IC50 and percentage of hydroxyl radical inhibition were determinedusingthemethodsdescribedabove.
Ina nutshell,eachplantextract(8192g/mL)was diluted2-foldwithMHBinatotalamountof100Lina96well microplate. Plant extract concentrations varied from 4096 ng/mL to 2 ng/mL. Once the microplate had been incubated at 37°C for 24 hours (bacteria) or 48 hours (yeast),100Lofinoculum(1.5106CFU/mLforbacteriaand 1.5 104 CFU/mL for yeast) was added to each well. The conventional medications (ciprofloxacin or ketoconazole) wereaddedtoonesetofwells,whiletheothersetservedas anegativecontrolandincludedbacteriaorfungus.Eachwell was filled with 40 L of INT solution (0.2 mg/mL), and the microplatewasincubatedat37°Cfor30minutes.Theyellow dye of INT is changed to a pink hue when it comes into contact with living bacteria or yeast. The MIC was determinedbynotingtheconcentrationofextractneededto avoid a detectable color change in the medium. After incubation for the MIC test, wells that did not indicate growthwerere-inoculatedwith50Lofthetestorganism, and the resulting volume was added to 150 L of MHB (bacteria)orSDBtodeterminetheMBCorMFC(yeast).The microplatewasthenkeptina37°Cincubatorfor48hours. The minimal bactericidal concentration (MBC) and the minimalfungicidalconcentration(MFC)weredeterminedby testing a variety of extracts against various microbial populations.Theexperimentwasrunthreetimes,eachtime withaduplicatesetofresults.
Table 1: Thestudy'smedicinalplants'definingfeatures.
Scientific Name Part Used Traditional Used Previous Pharmacol ogical Studies
H.Docum ents Leave EyeInfection sprain,female infertility, trypanosomias sssis,hernia, beriberiand gastralgia.
L.Macroc arpa Fruit Genital stimulants/de pressants, aphrodisiac
T.Mauriti anum Leave Wounds, cough,and premenstrual tension
Isolated Phytoch emical compou nd
Not reported Not reported
C.Stellulif erum Whol e Plant
A.Mannia na Leave seeds , Stem bark
Amnionitis affectingthe newborn, polyh ydramnios
acidand sitosterol
Immunomo dulatory Tannins
Filariasis Antioxidant Flavonoi ds, quinones, tannins, terpenoid s,and steroids
C.Boughe yanum Whol e Plant
Notreported Acuteand sub-chronic toxicity
P.Pedunc ularis Leave Heart conditions [26]toothache, convulsion, yellow jaundice, stomachache,
Not reported Not reported
Antisalmone llaland antioxidant
2,4-ditertbutylphe nol2 ((octylox y) carbonyl) benzoic
Not reported
Not reported Not reported
Phytochemical Analysis: Seven medicinal plants' methanolicextractswereanalyzedfortheirphytochemical content,andthefindingsaresummarisedinTable2.Allthe plantextractswerefoundtohavephenols,flavonoids,and steroids.Exceptforanthraquinone,everyphytochemicalwas presentintheL.macrocarpaextract.Inaddition,allplants contained saponins, except for A. manniana and P. peduncularis.
Total Phenolic and Flavonoid Contents.: Figure 1 displaysthephenolicandflavonoidcontentsofavarietyof medicinal plants. P. penduncularis and T. mauritianum extractshadthegreatesttotalphenoliccontent(5.570.22 mgGAE/gand4.920.55mgGAE/g,respectively).Extractsof C. bougheyanum and H. decumbens, on the other hand, showedthelowestTPC(0.790.06mgGAE/gand0.480.05 mgGAE/g,respectively).TFCwasgreatestforL.macrocarpa (0.110.01mgQE/g)andlowestforP.peducularis(1.380.06 mgQE/g).SimilartotheA.mannianaextract(0.390.04mg QE/g),theTFCoftheC.stelluliferumextractwasalso0.36 0.02mgQE/g.
Antioxidant Activity.: Tabulated in Table 2 are the resultsofDPPH,OH,andNOradicalscavengingexperiments used to assess the antioxidant properties of extracts from medicinalplants.TheplantextractshadIC50valuesbetween 0.55and49.43g/mLfortheDPPHand0.65and13.7g/mL
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072
for the NO assays. The IC50 values of the P. peduncularis extractwerecomparabletoascorbicacidwhentestedusing theDPPHandNOtechniques.
Table-2: Sevenmedicinalplants'phytochemicalsin methanolextractswereanalysedqualitatively.
Phytochemical groups Plant extracts Hd Lm Tm Cs Am Cb Pp
Alkaloids - + - + - +Phenols + + + + + + + Flavonoids + + + + + + + Saponins + + + + - +Triterpenes + + - - + - + Steroids + + + + + + + Anthraquinone - - + - + -Tannins + + + + + - +
Note: +: the presence of phytochemicals, − : absence of phytochemicals, Hd: H. decumbens,Lm: L.macrocarpa,Tm: T. mauritianum, Cs: C. stelluliferum, Am: A. manniana, Cb: C. bougheyanum, Pp: P. peduncularis.
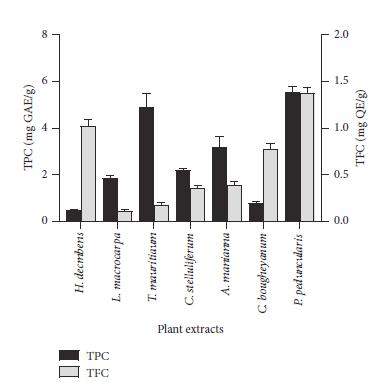
Table 3: IC50 (μg/mL)valuesofsevenmedicinalplant extractsagainstDPPH,OH,andNOradicalscavenging. IC50 (μg/mL)
DPPH OH NO H. decumbens 35.07±0.55 123.59 •± 0.23 10.44 •± 0.36 L. macrocarpa 49.43±0.06 ˃1000 0.78 •± 0.00 T. mauritianum 25.88 •± 0.54 169.82 •± 0.30 13.7±0.81 C. stelluliferum 58.88±0.59 79.06 •± 0.80 5.15 •± 7.07 A. manianna 37.15±0.86 153.46 •± 1.94 7.34 •± 0.13 C. bougheyanum 30.97 •± 0.10 67.29 •± 0.55 5.5 8 •± 0.06 P. peduncularis 0.55 •± 0.00 512.86 •± 0.93 0.60±0.00 Ascorbicacid 0.45 •± 0.00 52.6 •± 0.35 0.52 •± 0.00
Figure 1: Thetotalphenoliccontent(TPC)andtotal phenoliccontent(TFC)ofsevenextracts.
Moreandmorenationsarereportingthattheircitizensare using medicinal plants for their pharmacological effects. More than 25% of pharmaceuticals are produced from plants, according to the World Health Organization [12]. Phenols,flavonoids,andsteroidswereallidentifiedbythe phytochemicalanalysisashavingkeybiologicalrolesinthe current investigation [28]. Anti-inflammatory, antispasmodic, antiulcer, antidepressant, antidiabetic, cytotoxic,antitumor,antibacterial,andantioxidantactions areonlysomeofthemanybenefitsassociatedwithphenolic and flavonoid chemicals. Another benefit of therapeutic plantsteroidsistheirabilitytokillgermsandinsects[27]. Flavonoids,quinones,tannins,terpenoids,andsteroidswere all discovered in A. manniana by Ngbolua et al. [24], thereforeourfindingsareconsistentwiththeirfindings.In addition, Wickens and Burkill, using a similar financing model,demonstratedthepresenceoftanninsinanextractof C. stelluliferum [21]. Saponins were found in every plant tested except for C. stelluliferum and P. peduncularis. As statedin[28],saponin-containingplantextractshavebeen used to treat inflammation, cerebrovascular and cardiovasculardisorders,stomachulcers,andUVdamage. Saponinshavealsobeenutilizedasadjuvantstoimprovethe bioavailabilityofbioactivecompoundsandpharmaceuticals [29]. This study's plant extracts may have been used as a traditionalmedicinebythepeopleoftheTombelsubdivision sincetheycontaincertainphytochemicalcomponents.
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056

Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072
Select therapeutic plants' total phenolic and flavonoid contents were also studied. The TPC and TFC levels were greatest in the P. penduncularis extracts. This plant may have enhanced biological capabilities due to its highconcentrationsofphenolicandflavonoidchemicals.No onetestcandefinitivelyestablishantioxidantactivity[40]. DPPH, OH, and NO radical scavenging tests were used to ascertain the antioxidant activity of the medicinal plants under investigation. Very strong antioxidant activity is defined as an IC50 value of 50 g/mL or less, strong antioxidant activity as 50 g/mL to 100 g/mL, moderate antioxidant activity as 100 g/mL to 150 g/mL, and weak antioxidant activity as 150 g/mL or more [41]. This data demonstratesthatallplantextractstestedhadhighlevelsof antioxidant DPPH and NOradical scavenging activity. Further, IC50 values of 79.06 g/mL and 67.29 g/mL were found for C. stelluliferum and C. bougheyanum extracts, respectively, demonstrating potent OH scavenging action. Medicinal plants analyzed may have shown antioxidant actionduetothepresenceofphenoliccomponentsincluding phenolicacidsand flavonoids.These phenoliccompounds areeffectiveantioxidantsbecauseofthehydrogen-donating abilities of the hydroxyls in their phenolic groups [12]. In addition,themetalionsthatcontributetoROSgeneration maybechelated byphenolic compounds [13].Similarities existbetweenourfindingsandthoseofNgboluaetal.[24], whoalsofoundthatA.mannianahasantioxidantproperties. ItwasalsofoundbyTsafacketal.[17]thatT.mauritianum hasantioxidantproperties.
The findings of this research showed that the medicinal plantsexaminedhaveantibacterialandantifungalcapability againstdrug-resistantinfections.Thesemedicinalherbsalso have the potential to be utilized as an organic antioxidant supplement. The mechanism of action and potential lead moleculesforthedevelopmentofnovelmedicationsmaybe determinedifthebioactivechemicalsintheseplantextracts werefurtherpurifiedandisolated.
[1] K. Iskandar, J. Murugaiyan, D. Hammoudi Halat et al., “Antibioticdiscoveryandresistance:thechaseandtherace,” Antibiotics,vol.11,no.2,p.182,2022.
[2]S.Basak,P.Singh,andM.Rajurkar,“Multidrug-Resistant and extensive drug-resistant bacteria: a study,” Journal of Pathogens,vol.2016,ArticleID4065603,5pages,2016.
[3] WHO, Antimicrobial Resistance, World Health Organization,Geneva,Switzerland,2022.
[4]Z.Liu,Z.Ren,J.Zhang,etal.,“RoleofROSandnutritional antioxidantsinhumandiseases,” Frontiers inPhysiology,vol. 9,p.477,2018.
[5] M. A. Chelombitko, “Role of reactive oxygen species in inflammation:aminireview,” Moscow University Biological Sciences Bulletin,vol.73,no.4,pp.199–202,2019.
[6]H.J.FormanandH.Zhang,“Targetingoxidativestressin disease: promise and limitations of antioxidant therapy,” Nature Reviews Drug Discovery,vol.20,no.9,pp.689–709, 2021.
[7] S. Mansoor, O. Ali Wanie, J. K. Lone, et al., “Reactive oxygenspeciesinplants:fromsourcetosink,” Antioxidants, vol.11,2022.
[8] T. D. Oluwajuyitan, O. S. Ijarotimi, and T. N. Fagbemi, “Plantain based dough meal: nutritional property, antioxidantactivityanddyslipidemiaamelioratingpotential inhigh-fatinducedrats,” Clinical Phytoscience,vol.7,no.1,p. 92,2021.
[9] T. Khare, U. Anand, A. Dey et al., “Exploring phytochemicals for combating antibiotic resistance in microbial pathogens,” Frontiers in Pharmacology, vol. 12, ArticleID720726,2021.
[10]N.Vaou,E.Stavropoulou,C.Voidarou,C.Tsigalou,andE. Bezirtzoglou, “Towards advances in medicinal plant antimicrobial activity: a review study on challenges and futureperspectives,” Microorganisms,vol.9,no.10,2021.
[11]B.N.Bisso,P.N.Kayoka-Kabongo,R.T. Tchuenguem, and J. P. Dzoyem, “Phytochemical analysis and antifungal potentiating activity of extracts from loquat (Eriobotrya japonica)against Cryptococcus neoformans clinicalisolates,” Advances in Pharmacological and Pharmaceutical Sciences, vol.2022,ArticleID6626834,6pages,2022.
[12]A.Rasool,K.M.Bhat,A.A.Sheikh,A.Jan,andS.Hassan, “Medicinalplants:role,distributionandfuture,” Journal of Pharmacognosy and Phytochemistry,vol.9,no.2,2020.
[13]S. Savadi,M. Vazifedoost,Z.Didar, M.M.Nematshahi, and E. Jahed, “Phytochemical analysis and antimicrobial/antioxidantactivityof Cynodon dactylon (L.) pers.rhizomemethanolicextract,” Journal of Food Quality, vol.2020,ArticleID5946541,10pages,2020.
[14] K. K. U. Mbuta and P. Latham, Plantes m´edicinales de traditions province de l’Equateur–R.D. Congo IRSS(Institut de Recherche en Sciences de la Sante), Kinshasa, Democratic Republic of the Congo,2ndedition,2012.
[15] M. O. Soladoye, E. C. Chukwuma, J. O. Ariwaodo, G. A. Ibhanesebor,O.A.Agbo-Adediran,andS.M.Owolabi,“Our plants,ourheritage:preliminarysurveyofsomemedicinal plant species of Southwestern University Nigeria Campus, Ogun State, Nigeria,” Scholars Research Library Annals of Biological Research,vol.4,no.12,2013.
International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056 Volume: 09 Issue: 11 | Nov 2022 www.irjet.net p-ISSN:2395-0072

[16] M. Saive, M. Frederich, and M. L. Fauconnier, “Plants usedintraditionalmedicineinthecomorosarchipelago.A review,” Biotechnology, Agronomy, Society and Environment, vol.24,no.2,pp.117–141,2020.
[17]D.N.Tsafack,N.Kodjio,G.S.S.Nateng,C.Fokunang,and T.D.Sedric,“In vitro antisalmonellaandantioxidanteffects of various extracts from leaves and stem of Tristemma mauritianum (Melastomataceae),” Research Journal of Pharmaceutical, Biological and Chemical Sciences,vol.8,no. 3,2017.
[18]N.Tsafack,A.F.Yameen,G.S.S.Natengetal.,“GC/MS analysis,antisalmonellalpotentialofmethanolleafextracts of Tristemma mauritianum and effects on hematological parametersonWistarratsinfectedwith Salmonella typhi,” International Journal of Pharmacy,vol.7,no.2,pp.120–131, 2017.
[19] D. Njamen, M. A. Mvondo, S. Djiogue, G. J. M. Ketcha Wanda,C.B.MagneNde,andG.Vollmer,“Phytotherapyand women’s reproductive health: the Cameroonian perspective,” Planta Medica, vol. 79, no. 7, pp. 600–611, 2013.
[20] J. N. Nfozon, M. O. Kamtchueng, R. Nkwelle et al., “Evaluationofthe in vitro immunomodulatoryactivityoflic extracts of Triplotaxis stellulifera (BEUTH) HUTCH. and Crassocephalum vitellinum (BENTH)S.Moore,” International Journal of Agriculture Environment and Biotechnology,vol.6, no.1,pp.41–53,2021.
[21] G. E. Wickens and H. M. Burkill, “The useful plants of west tropical Africa,” Kew Bulletin, vol. 41, no. 2, p. 471, 1986.
[22] D. A. Focho, W. T. Ndam, and B. A. Fonge, “Medicinal plantsofAguambu BamumbuintheLebialemhighlands, southwest province of Cameroon,” African Journal of Pharmacy and Pharmacology,vol.3,no.1,2009.
[23] C. W. Choi, S. B. Song, J. S. Oh, and Y. H. Kim, “Antiproliferation effects of selected Tanzania plants,” African Journal of Traditional, Complementary and Alternative Medicines,vol.12,no.2,p.96,2015.
[24] K. Ngbolua, K. S. Dalley-divin, M. M. Jean, K. K. Jeanclaude,andK.K.Odilon,“Phytochemicalinvestigationand TLC screening for antioxidant activity of 24 plant species consumedbytheEasternLowlandGorillas(Gorillaberingei ssp.graueri:hominidae,Primates)endemictoDemocratic RepublicoftheCongo,” Journal of Advancement in Medical and Life Sciences,vol.1,no.3,2014.
[25] J. N. Nfozon, C. Tume, N. Kdjio et al., “Acute and subchronictoxicityevaluationof Triplotaxis stellulifera (Benth) Hutch and Crasssocephalum bougheyanum C. D. Adams
methanol extract on mice,” Biochemistry and Analytical Biochemistry,vol.8,no.3,pp.1–10,2019.
[26]J.O.Odukoya,J.O.Odukoya,E.M.Mmutlane,andD.T. Ndinteh,“Ethnopharmacologicalstudyofmedicinalplants usedforthetreatmentofcardiovasculardiseasesandtheir associatedriskfactorsinsub-SaharanAfrica,” Plants,vol.11, no.10,p.1387,2022.
[27] L. C. Hwang, H. R. Juliani, R. Govindasamy, and J. E. Simon, “Traditional botanical uses of non-timber forest products (NTFP) in seven counties in Liberia,” ACS Symposium Series,vol.1361,pp.3–43,2020.
[28] J. B. Harborne, Phytochemical Methods A Guide To Modern Tecniques Of Plant Analysis, Chapman and Hall, London,UK,3rdedition,1998.
[29] J. P. Dzoyem and J. N. Eloff, “Anti-inflammatory, anticholinesteraseandantioxidantactivityofleafextractsof twelve plants used traditionally to alleviate pain and inflammation in South Africa,” Journal of Ethnopharmacology,vol.160,pp.194–201,2015.
[30] K. Sowndhararajan and S. C. Kang, “Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii wight and arn,” Saudi Journal of Biological Sciences,vol.20,no.4,pp.319–325,2013.