International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072
1PG Scholar, Department of Civil engineering, Bapuji Institute of Engineering and Technology, Davanagere, Karnataka, India
2Professor and Head, Department of Civil Engineering and Technology, Davanagere, Karnataka, India ***
Abstract – Leachate and soil samples were taken for this study from the Davanagere city landfill site near Avaragolla. The purpose of the study is to evaluate the physical and chemical elements and the concentrations of heavy metals in the leachate and surrounding soil of the landfillsite. The dump at Avaragolla Village is located in Davangere City, Karnataka, about 14 kilometers away. Standard techniques were used to test the physicochemicalcharacteristics ofthe leachate sample on site, and heavy metals were evaluated using a Shimadzu AA7001 Atomic Absorption Spectrophotometer following nitric acid digestion. The majority of the determined heavy metal values were over the recommended limits. Inthe current study two soil types silty sand and clayey sand were selected. Soil Samples were collected at 4 different locations and depth in and around the landfill site. The results indicated that the heavy metals, namely Cu, Cr, Fe and Zn were significant concentrations in the soil within a radius of1000m from the landfill. Zn > Fe > Cr > Cu was the order of the heavy metal concentration in the soil sample that was obtained. Leaching column studies were carried out to setup the development curve, which showed that heavy metals (Cu, Cr, Fe, and Zn) were retained in clayey and silty sand soils.
Key Words: MunicipalSolidwaste,Landfill,Leachate,Heavy metals,Soil.
ThedistrictofDavangereislocatedatthecentralpartof theKarnataka,lodginganareaof5,924km²andthecurrent metroregionpopulationofDavangerein2022is530,000,a 1.73%enhancedfrom2021.Thedistrictproducesveryhigh municipalwasteproduction168.32TPDofwasteassessedto be around 1.2kg per person every day (Shravan and Nagarajappa,2018).Inadditiontowastematerialsthatenter landfillsbypercolationwithground-waterinternalflowor through penetration from rainfall, landfill locations are regarded as a major hazard to ground-water resources. (Clarke,etal.,2015).

Thecurrentworkhighlightingontheseproblemsisrare, so in direction to take up adequate safety protection and upgraded standards. It is energetic that appraisal of the effects of polluted leachate on, physical - chemical characteristics of the natural soil in and around the
Davangere city takes place. In this work, a detailed laboratoryappraisalwasassumedtoappraisetheeffectsof land-fillleachatepollutiononthepropertiesofnaturalsoils ofinandarounddumpingsiteofDavangere.
The chief objective of this work is to appraise the performanceoftheAvaragollaVillage,withanopiniontoaid future and construction of land-fills in Davangere. The objectivesoftheworkare:
To appraise the impact of leachate on propertiesofsoilsatsolidwastedumpingsite ofDavangere.
Toexplorethefactoftheleachateindifferent soilsamplessurroundingatthedump-site.
Illustrate and distribution of trace metal elementsinland-fill.
Fig -1.1:Avaragollalandfillsite
For this work, soil samples were taken from a landfill (Avaragolla Village, average area is 33 acres). As
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072
showninFig.-1.1,theland-fillsiteislocatedaround14km south-west of Davangere city. The layer of the Avaragolla village are formed mostly by two types; Silty and Clayey sand soil samples of which were attained from earlier researchers(Jeragh2009,2012). Inthevillage,atwosquare kilometer stretch of land has been adopted as a trash disposalsite.
Soilssamplingatthreedifferentseasons,depths,inandaroundtheland-fillincludedandcontaminatedpartsof thesite.Thefirstwasdoneon12-13December,2021during drywinterperiod.Thesecondsamplingwasdoneon14-15 May2022duringtopdryseason.Thefinalsetsofsamples werecollectedon20-21June2022duringrainyperiod.
The aerial picture of Avaragolla Village, which is coveredbytheland-fillareaanditssurroundingregion,four soil samples were taken at a depth of 10 cm below the surfaceoftheground.Thevaluesofsamplingappraisalwere adoptedforthecurrentstudy.
The second sampling was completed. The 2 soil samples were taken. Samples were collected at a below depth10-20cmateachpointfromrandomgenuinegridona circle with in successive radius of 500, 1000 and 1500 m awayaftertheland-fillarea.
CollectedsoilsamplesfromLand-fillsite,weredried andgrindedsoilpassthrougha2mmstainlesssteelsieveto separates gravel and rock. For analysis, homogenized soil sample is collected. Physico-chemical variables were determined pH, EC (Conductivity meter), Organic Matter, Cation exchange capacity. For metal analysis soil was digestedusingnitricacidmeasuredthetracemetals(Fe,Zn, Cr and Cu) using Shimadzu AA7001 Atomic Absorption Spectrophotometer). Statistical analysis was performed usingSPSS10.0forWindowstounderstandthesignificant relationshipwithinthevariables.Thecorrelationsbetween soilvariablesandformetalswerealsoevaluated.Thesoils adopted in the laboratory analysis were neutral soils collected from test pits of 0.6 to 3.0 m depth of the Averagolla land fill area. The collected samples from the selectedlocationwerecleanandthesoilswereclassedatthe civil engineering laboratories of BIET in accordance with ASTMcriteria
Particlesizewasdeterminedforthreedifferentsoil samplesusingthemethodoutlinedinASTMD422(2007a), which was applied to laboratory testing using 750g of washedclayeysoiland350gofsplashedsiltysand.Nos.4, 10, 100, and 200 of the ASTM standard sieve were used. Accordingtothemodifiedmethod(ASTMD15572012a),the soils' maximum dry compactness and ideal moisture percentagewereassessed,andthefielddensityofthesoil sampleswascalculated(ASTMD1556,2007b).Thespecific
gravity (Gs) of the soil was evaluated using the moisture percentageofthesoil,whichwascalculatedandreportedas a percentage using a frame of dry soil and water present (ASTMD2216,2010b)(ASTMC128,2012b).Thechemical propertiesofthecollectedsoilssamplesaremeasuredfor pH(ElectrometricmethodBS1377part1(1990),organic matter(BS1377part1(1990).
The final sampling 2 soil locations significantly polluted by leachate was sample at few depths. This final samplingwasplannedtoattaintheresultsofcontaminants. Thesoilsamplesweredesignatedfromsignificantcontentof thetracemetalsintheselectedsite.Thechemicalproperties ofthecontaminatedliquidwasteappraisedinthisstudy.The heavy metals content was determined by using Shimadzu AA7001AtomicAbsorptionSpectrophotometer.
Adsorption Isotherms study was conducted to estimatethecommunicationbetweentheleachateandsoil (USEPA,2010).Thesoilssampleswereairevaporatedfor1 day, then crushed up adopting crusher then sieved using 2.0mmsize.Eachofthefourheavymetalsolutions(copper, iron, zinc, and chromium), weighing around 250 mL, was purchased.Fiveratiosofsoilsolution 1:5,1:10,1:30,1:60, and1:100 weremadeforeachheavymetalliquidsolution thatwaschosenandstoredinpolyethylenecontainerswith closed lids. The maximum amount of heavy metals in the chosenleachateinformedthechoiceofliquidsolutions.
The hypothesis of the adsorption isotherms lines was applied using the linear Langmuir and Freundlich equation. According to the USEPA (2010), The linear Langmuirequationwrittenas: whereKLand
Marecoefficientscalculatedfromtheangleandequallinear balance. According to the USEPA (2010), the linear Freundlich balance can be written as: wherexisamountofthesoluteadsorbed,mistheamountof adsorbent (oven-dried soil), C is amount of solute equilibrium(Kf),and1/n=constantsderivedfromtheslope andequallinearequation.
Theinitialcontentsoftheheavymetalsandthree chemicalvariablesoftheleachatecollectedfromAvaragolla village landfill surroundings was appraised are given in (Table2.1)
Sl No Variables Results 12-13 Dec 2021
14-15 May 2022
20-21 June 2022
1. Ph 8.34 8.31 8.72
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072
2. EC 14.62 ms/cm 15.23 ms/cm 18.42 ms/cm
3. TDS 11704 mgL 12894 mg/L 13456 mg/L
4. Fe 4.38 mg/L 4.56 mg/L 5.01 mg/L
5. Zn 13.56 mg/L 14.2 mg/L 15.21 mg/L
6. Cr 0.32 mg/L 0.46 mg/L 0.56 mg/L
7 Cu 0.13 mg/L 0.16 mg/L 0.19 mg/L
Table-2.1. ChemicalpropertiesofCollectedLeachate sample
The pH values of the collected soil samples are alkalineinnaturewhichindicatespresenceofhighcalcium carbonateconstituents(Ismaeletal.1986).Thetotalorganic concentrationsofthesoilsareverylessthan1%.Caravaca andAlbaldejo(1999)andIsmaeletal.(1986)notedthatthe low precipitation and high temperatures in a semi-arid climate may be reducing the contribution of organic materials(Table2.2).
Type of Soil pH Calcium Carbonate (%) Organic Matter (%)
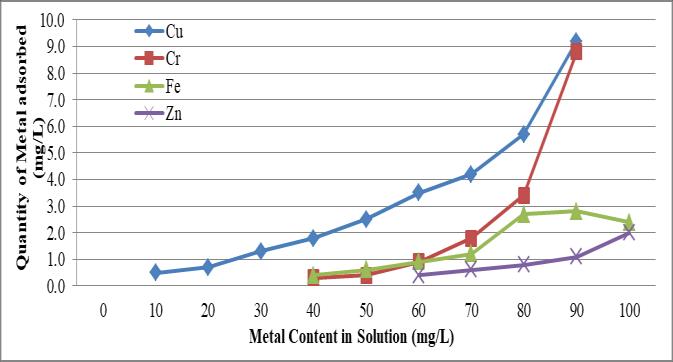
Fig - 2.1: Adsorptionofheavymetalsbysiltysandsoil
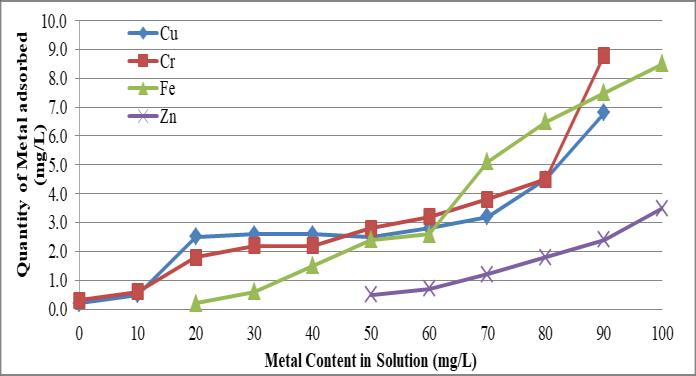
Figure 2.2 shows the amount of heavy metal adsorption in clayey soil. The amount of heavy metal adsorptionincreasedastheliquid'smetalcontentincreased. In the original content, over 96% of the Cu and Cr in the liquidwereadsorbed;however,only65.85%oftheFeand Zn were individually adsorbed. Maximum levels of metal adsorptionasrelatedtothesiltysandsoilwereshownby theclayeysand.ItispredictedthatalloftheCrandCuwill adsorbatlowconcentrations.Thiscanbecreditedtotheclay elements'tendencytoscatteratlowerconcentrationsdueto thefullexpansionofdiffusedoubledeposits,whichimproves theinteractionbetweenthesuperficialclayelementsandthe solution(Mohamedetal.1992).
Silty sand Soil
9.24 7.14 0.041
8.51 8.20 0.028 Clayey sand Soil
Table-2.2 AveragechemicalpropertiesofAvaragolla Villagesoilsample
The atomic absorption spectrophotometer, which was previously mentioned in this work, was used in the batch adsorption investigation to measure the amount of heavy metals adsorbed by soil for different soil solution ratios. To determine whether or not processes follow Langmuir/Freundlichisotherms,theadsorptiondatacanbe fittedusingtheadsorption equation Fig.2.1indicates the amount of metal adsorbed in the silty sand soil to the amountofmetalpresentinliquid.AccordingtoFig.2.1,the amountofheavymetaladsorptionincreasedastheamount ofmetalinthesolutionincreased,asshownbytheshadow parallellinesinthegraph.Atthebeginningoftheanalysisat lowmetallevelsinliquid,Cuadsorptionisgreaterthanthat of other metals. Although Zn's adsorption is smaller than that of Cr, Cu and Fe's adsorption were negligible. The highestamountofCradsorptiondependsmostlyonthesoil pHlevel,andCrliquefieswellinbothacidicandalkalinesoil (Wyszkwska2001).
AdoptingtheLangmuirandFreundlichequationsclarified therelationshipbetweentheliquidcontentandadsorption. TheLangmuirisothermwasrecognizedasbeingdependent on thermodynamic equilibrium. It is extensively used because of its simplicity and capacity to use a variety of adsorption facts. Plotting the amount of soil adsorbed (x) andtheamountofsoluteadsorbed(m)asanaccumulation of the equilibrium solute content allows for the determinationofthevariablesinLangmuirbalance(C),The slope was used to get the variables for the Langmuir constants(b,K),wherebrepresentsthegreateradsorption andKrepresentstherelationshipenergyoftheadsorbent's adsorption. The variables in Freundlich balance can be estimatedbyplottinglog(x/m)againstlog(C).
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072
TheFreundlichconstantvariables(Kf,n)appraisedfromthe slopeandinterruptofthelinearbalanceduetoadeficiency and lack of self-regulating mark regarding the definite preservationapparatus(Bucheretal.1989). However,the model introduces a number of conventions, such as comparable adsorption locations (which denotes that the adsorption locations are equivalent) and a mono-layer of adsorbents (the model proposes a highest of one layer of adsorption, but the in circumstance of clayey soil greater thanoneisprobable).Thelineardegradation(R2)valuesare usedasameasureofhowwelltheadsorptionfactsclose-fit. Fig-2.3 and 2.4 indicates the plot of the Langmuir and FreundlichgraphsforasiltysandsoilFig-2.5and2.6shows theanalyticalvaluesforasampleofclayeysandsoil.
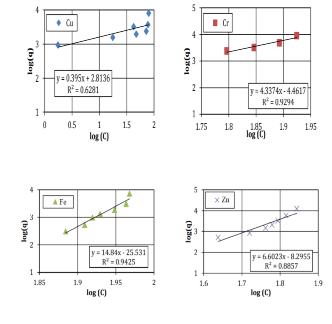
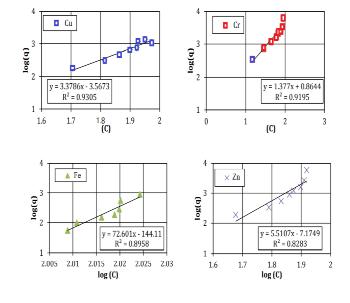
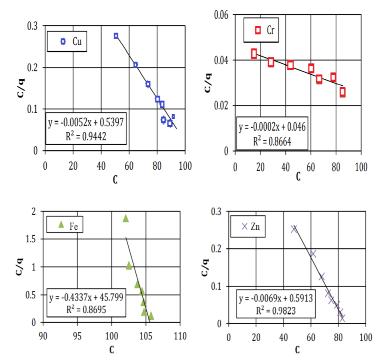
Fig-2.3Langmuirgraphsforasampleofsiltysandsoil
Fig-2.5Langmuirgraphsforasampleofclayeysandsoil
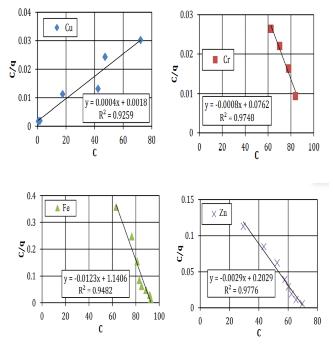
Fig-2.4Freundlichgraphsforasampleofsiltysandsoil
Fig-2.6Freundlichgraphsforasampleofclayeysandsoil
Table 2.3 contains a prediction of the analytical resultsofthelinearregressionproducedbytheFreundlich andLangmuirmodel.Forafewchosensoilsamples,itcanbe stated that the linear regression findings from the FreundlichandLangmuirmodelsarequiteacceptable..The R2 numericalforallsamplesfrom0.80and0.95,expectfor theCufortheclayeysoilforFreundlichmodel,whichis0.61.
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072
Freundlich Langmuir
Samples Cu Cr Fe Zn Cu Cr Fe Zn R2 Values
Silty Soil 0.92 0.91 0.89 0.81 0.92 0.85 0.87 0.94
Clayey Soil 0.61 0.92 0.94 0.88 0.92 0.97 0.93 0.96
Table 2.3 ThelinearregressionattainedfromFreundlich andLangmuirequation.
Thefindingsfora selectedsoilsamplesshowvery littlecapacitytoholdheavymetal.TheLangmuirvariables deliberatethehighestadsorption(b)andtheconnectionof energy adsorption The fact that the elements in the silty sand keeps neutral electrical responsibility and have minimalcation-exchangecapacitycanbeattributedtothe fact that the silty sand soil sample exhibited insignificant adsorptionandattachmentenergyforalltestedmetals.The soil samples from the clayey sand have high Cr and Cu adsorptions.
Samples
Freundlich Langmuir Silty Clayey Silty Clayey
Variable Kf N Kf n K b K B
Cu3.48 0.31 2.16 2.520.001 -191 0.110 5001
Cr 0.88 0.72 -4.42 0.220.1053323 0.003 1248
Fe143 -2.224.50 0.070.001 -2.2025.4881.20
Zn7.18 0.17 -8.20 0.150.014 -1420.012 -342
Table 2.4 ThevariablesofFreundlichandLangmuirfor selectedsoilsamples
Themaingoalofthisresearchwastoevaluatethe effects of landfill leachate on the clean soil and the local ecological system in Avaragolla Village, Davangere. The analytical results foreseen in this study are evaluated in relation to the geoenvironmental characteristics of particularsoilsandthepromotionofleachate.Theresearch shedlightonthelandfillsiteinthevillageofAvaragollaand how the disposal of solid waste there contributes to the contamination of the groundwater and nearby clean soils. Forthecurrentstudy,twonaturalsoilsamples(clayeysoil andsiltysoil)wereselectedsincetheybothcharacterizethe collectivesoilsintheAvaragollavillageandarecommonly
usedtorefertothem.Theleachatewascomposedfromthe Avergollavillagelandfillsite.
Thebasicphysicalcharacteristicsoftheselectedsoil samples were estimated by standard laboratory methods previous to the chief analytical program. According to reports, leachate has no effect on the silty sand soil that predominatesinAvaragollavillage.
Todeterminethecollected soils'abilitytoadsorb heavymetalsthatwillaffectthesoillayers,batchadsorption andcolumnanalysiswereundertaken. Theanalyticalvalues fromthegeo-environmentalanalysisareharmoniouswith the analytical values attained for the geo-technical characteristicsofthesiltysandsoil.Theoccurrenceofclay mineralsshowsasignificantroleintheclayeysandsoiland indicatesthesignificanceofcaptionsconversationinthesoil characteristics.Goodassessmentwasattainedbetweenthe laboratorytestresults.
1. Ali M.M.L., Ali M.M.L., Islam M.S., Rahman M.Z. (2016).Preliminaryassessmentofheavymetalsin water and sediment of Karnaphuli River, Bangladesh. Environmental Nanotechnology, Monitoring & Management, 5, 27–35, https://doi.org/10.1016/j.enmm.2016.01.002
2. Al-Muzaini, S. 2006. Characteristics of leachate at the Qurain dumping site. Journal of Food, Agriculture&Environment,4(2),pp.251-54.
3. ASTM Standard. 2006. D 4874 – 06 Standard test method for leaching solid material in a column apparatus. West Conshohocken, PA: ASTM International.
4. Banerjee, M., Bar, N., Basu, R. K. & Das, S. K. 2018 RemovalofCr(VI)fromitsaqueoussolutionusing greenadsorbentpistachioshell:afixedbedcolumn study and GAANN modeling. Water Conservation Science Engineering 3 (1), 19–31. https://doi.org/10.1007/s41101-017-0039-x.
5. Caravaca,F.,Lax,A.andAlbaladejo,J.1999.Organic Matter, Nutrient Contents and Cation Exchange Capacity in Fine Fractions from Semi-arid CalcareousSoils.Geoderma,93(3-4),pp.161-76.
6. Chinade A.U., Umar S., Osinubi K. (2017) Effect of municipal solid waste leachate on the strength of compacted tropical soil for landfill liner, InternationalResearchJournalofEngineeringand Technology,4(6),3248–3253.
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072
7. N.U. (2019). Ecotoxicological status and risk assessment of heavy metals in municipal solid wastes dumpsite impacted soil in Nigeria, Environmental Nanotechnology, Monitoring & Management,11,https://doi.org/10.1016/j.enmm.2 019.100215.
8. FongeB.A.,NkolekaE.N.,AsongF.Z.,AjoninaS.A., &CheV.B.(2017).Heavymetalcontaminationin soils from a municipal landfill, surrounded by banana plantation in the eastern flank of Mount Cameroon.AfricanJournalofBiotechnology,16(25), 1391–1399. https://doi.org/https%3A//doi.org/10.5897/ ajb2016.15777.
9. Kanmani,SandR.Gandhimathi.2013.Assessment ofheavymetalcontaminationinsoilduetoleachate migrationfromanopendumpingsite,ApplWater Sci,3:193–205