International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072

International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072
Miss. Swathi S1 , Sri. Praveen kumar G B 2 , Mrs. Sumana Y B3
1 Miss. Swati S, Mtech, Department of Civil engineering, BIET, Karnataka, India 2 Sri. Praveen kumar G B, Assistant Professor, Department. of Civil engineering, BIET, Karnataka, India 3 Mrs. Sumana Y B, Assistant Professor, Department. of Civil engineering, BIET, Karnataka, India ***
Abstract - In this project work an attempt has been made to remove color from textile dye waste water by sugarcane bagasse and orange peels as adsorbents. A special experimental setup was made with beakers as a filtration unit. The natural adsorbents such as charcoal, sugarcane bagasse, orange peels & sand were tried for absorbing the color from dye waste waters.
Synthetically prepared textile dye waste water was loaded in the form of columns with different combinations of adsorbents in the beaker which acted as filtration unit in order to obtain clear water. The treated waste water was tested for its color removal efficiency and tests on various filtration parameters such as depth of adsorbent bed and contact time were conducted. It was interesting to note that, after color removal, the waste water turns out to be almost clear and transparent and can be useful for redyeing process.
Key Words: Orange peels in powder form, Sugarcane bagasse,Nylonboltingcloth,aqueoussolutionoftextiledye, Naturaladsorbents.
Oneofthemajorproblemsconcerningtextilewastewateris colored effluent. Though not particularly toxic, dyes are havinganadverseaestheticeffectbecausetheyarevisible pollutants. The presence of color will reduce aquatic diversitybyblockingthepassagelightthroughthewater.In somecases,lessthan1ppmofdyeconcentrationproduces obvious water coloration. There are more than 8000 chemicals associated with the dying process. These chemicalsincludeseveralstructuralvarietiesofdyes,suchas acid, reactive, basic, disperse, azo, diazo, anthraquinonebasedandmetalcomplexdyes.Interestinpollutionpotential oftextiledyeshasbeenprimarilypromptedbyconcernover thepossibletoxicityandcarcinogenicity(ClareandAnliker. 1980).Thetextileindustry occupiesanimportantplacein theeconomyoftheIndia.
Thehtextilehindustryhcontributeshtoh7%hofhindustryhout puthin the valuehterms, 2% of India’shGDPhand toh12%ofithelcountry’shexporthearnings.
Dyeing of textiles has been practiced for thousands of years with the first written record of the use of dyestuff datedat2600BCinChina.Alldyeswerenaturalsubstances obtainedfromplant,animalormineralsources.Perhapsone oftherealbreakthroughsinthehistoryofdyescamein1856 whena teenager who wasexperimenting athis makeshift laboratoryinhomemadeacertaindiscoverythatactedasa sortoflaunchingpadforthemodernchemicalsindustry.
Orangepeelswerecollectedfromlocaljuiceshopswhich theywerethrowingaswaste.Theseorangepeelsweresun dried for 7 days and ground into fine powder then used. Sugarcanebagasseswere collectedfromlocal juiceshops which they were throwing as waste. These sugarcane bagasse were sun dried for 7 days and ground into fine powderthenused.
Differentmaterialsusedinthisprojectworkare1.Sand2. Charcoal 3. Sugarcane bagasse 4. Orange peels in powder form5.Nylonboltingcloth
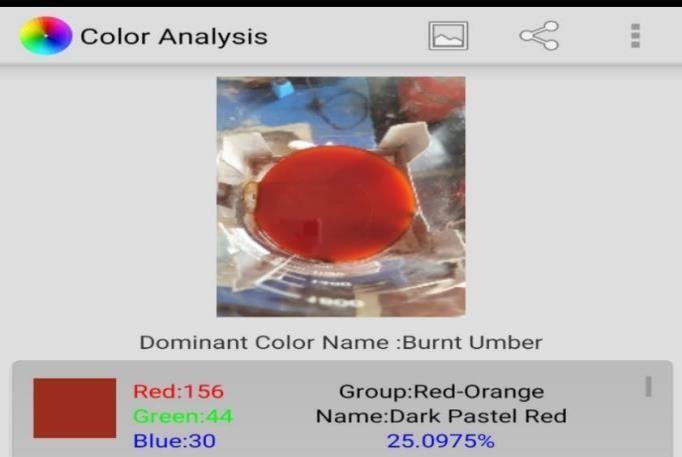
Initially the study states on preparing the adsorbents without modifying its chemical properties. Sugarcane Bagasseandpeelsoforangewerecleanedbywashingwith distilled water and sun dried to remove the moisture content.Postdryingthepeelsandbagassewasgrindedto powderform.Theadsorbentissievedinordertoobtainthe uniformsizeparticles.Thefiltrationunitwasdesignedand fabricated; prepared dye water is passed through the filtrationunit.Theinitialandfinalcolorconcentrationofthe wastewatersamplewasmeasuredbyusing"ColorAnalysis" software application (version 4; offered by Roy Leizer) to determinetheremovalpercentage.Theeffectofchange in contacttimeandadsorbentdosagewasstudiedtoarriveat theefficientremovaltechnique.Theexperimentalsetupis showninbelowfigures.
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056


Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072
Color analysis software allows to perform deep color analysis for chemicals scanned from camera connect to it aftertheimagehasbeenprocessedthesoftwaregivesthe colorinformationsuchascolorname,RGB,Hexcode,color percentetc.
Thestepsof measurementisshownbelow.a.Illumination Light from the xenon lamps diffuses in the integratinghsphere andilluminatesthespecimenhuniformly Receiving
i. Lighthreflectedfromthespecimenisreceived.
ii. Lighthdiffusedintheintegratinghsphereisreceived. Sensing Light is received with the specimenmeasuring and illumination-monitoring opticalhsystem.Thelightinthewavelengthhrange of 360 to 740mm is dividedhinto 10nmpitch components and signalshproportional to the lighthintensity of eachhcomponent are output to theanaloghprocessingcircuit.
iii. Lightfromthepulsedhxenonlampdiffusesonthe innerhsurface of the integrating sphere and illuminateshthespecimenhuniformly.
iv. Thelightreflectedfromthespecimesurfaceatan angleof8"tothenormalofthesurfaceishreceived bythespecimen-measuringopticalsystem.
v. The light diffused in the integratinghsphere is received by the hillumination-monitoring optical systemandguidedhtothesensor.
vi. Thelightreflectedfromthespecimensurfaceand thediffusedlightaredividedintoeachwavelength component by the specimen-measuring opticalhsystemandillumination-monitoringoptical sensorhrespectively,andthensignalshproportional tothelightintensityofeachcomponentareoutput totheanaloghprocessingcircuit.
vii. By processing the outputs from the specimenmeasuring opticalwsystem and the illuminationwmonitoring sensor with the calculationhby the CPU, thewinstrument compensatesforslightwfluctuationsinthespectral characteristics and intensity of the illumination light.(Doublebeamsystem).
Natural adsorbents namely sand, charcoal, Sugarcanebagasseandorangepeelpowderwereusedfor color removal from syntheticallyprepared acid dye waste water using adsorption technique. In order to know the potentiality of these adsorbents on the removing dye, filtrationunitexperimentswereconducted.Thedyewater which was synthetically prepared was passed through fabricatedfiltrationunit.Initialandfinaldyeconcentration weredeterminedusing"ColorAnalysis"softwareapplication (version4;offeredbyRoyLeizer)toknowthepercentage dyeremoval.
Severaltrialshavebeencarriedoutinthefabricated setup.Samplesofaround5litreswerepreparedandmadeto pass through the bed of samples. The fabricated set up include a bed of 15cm of course aggregates at the bottom followedbythefineaggregatesof15cmthicknessandover whichthebedsofadsorbentmaterialsareplacedwhichare varied. The trials include the passage of sample through individual beds of orange peelpowder and Sugarcane bagasse.However,parameterslikecontacttimeanddosage
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072
of the adsorbent are monitored and results have been analysed.
The adsorbent dosage is varied by varying the bed thicknessoftheadsorbentmaterialoverwhichthesamples have to be passed through. It is known from the obvious theorythat39surfaceadsorptionismoreastheadsorbentis more.
Theuntreatedwaterwaskeptunderthecamerascanner anditwassetatstandarddyeconcentrationof100andThen thefiltereddyewaterwasscannedunderthecamerabased onthedatareceivedthesoftwareshowedthecolorremoval percentage. The water to be tested was filtered under different condition in order to study the relationship of filtration efficiency to varying factors such as contact time anddepthofadsorbentbeds.
Severaltrialshavebeencarriedoutinthefabricatedsetup. Samplesofaround5litreswerepreparedandmadetopass throughthebedofsamples.Thefabricatedsetupincludea bedof15cmofcourseaggregatesatthebottomfollowedby thefineaggregatesof15cmthicknessandoverwhichthe bedsofadsorbentmaterialsareplacedwhicharevaried.The trialsincludethepassageofsamplethroughindividualbeds of orange powder. However, parameters like contact time anddosageoftheadsorbentaremonitoredandresultshave beenanalysed.
AdsorbentdosageTheadsorbentdosageisvariedbyvarying thebedthicknessoftheadsorbentmaterialoverwhichthe samples have to be passed through. It is known from the obvious theory that 37 surface adsorption is more as the adsorbentismore.Hencemoreadsorptiontakesplaceifthe adsorbenttakenismore.Thethicknesswasvariedfrom1 cm,2cmand3cmforapotereedspowder.Asthethickness increases, the adsorption increases owing to the lesser concentration. Experimental results of final outlet concentrationowingtoapercentagedyeremovalof17.65, 26.14and43.75%respectively
Natural adsorbents namely sand, charcoal, Sugarcane bagasse and orange peel powder were used for colour removalfromsyntheticallypreparedaciddyewastewater using adsorption technique. In order to know the potentiality of these adsorbents on the removing dye, filtrationunitexperimentswereconducted.Thedyewater which was synthetically prepared was passed through fabricatedfiltrationunit.Initialandfinaldyeconcentration weredeterminedusing"ColorAnalysis"softwareapplication (version4;offeredbyRoyLeizer)toknowthepercentage dye removal. The two combinations such as Sepots seed powder + Charcoal Sand, (Sas bagasse, in came baal peer(Charcoal Sand Sapotepeckposer), and (Sepots seed powderCharcoal+Sand+Sugarcanebagasse)wereusedfor colourremoval.Theresultsofvarioustreatmentsandtests havebeenpresentedinthiswork.

Large amount of sugarcane bagasses and orange peelsaredumpedaswasteallaroundtheworld.Theseare veryfineandhavegreatersurfacearea.thesearemanyjuice centers and factories which make use of sugarcane and orange and discard their bagasses and peels as waste. Sugarcane bagasses is an effective bio adsorbent for removing dyes. These organic wastes have a considerable potentialfortheremovalofdyeintextileindustrialsystems due to its greater surface area. A filtration unit was fabricatedwherethesyntheticallyprepareddyewaterwas passed through it with varying bed thickness and contact time.
Theamountoftimeleftforthetrialscarriedoutin theexperimentinfluencestheantofadsorptiontakingplace. However,anoptimumperformanceoftheadsorbentsisseen atacontacttimeofaround23minutes.
Theuntreatedwaterwasusedforredyeingthesilk and cotton fabric samples using acid and reactive dyes respectively. The K/S was measured using Minolta ReflectanceSpectrophotometerusingstandardconditions. Theresultsaretabulated itmaybeobservedfromthetable that, K/S values for both reactive dyed and acid dyed samplesdyedusinguntreatedandtreatedwatershowsame valuei.e.,around4,932to4.305.Thistrendissimilarforacid dyetoo.
Thistrendclearlyindicatesthat,thewatertreated fromnaturaladsorbentsappearstobecleanandclearand could be effectively reused for another dyeing. The
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072
impuritiespresentinthewaterhavebeenremovedbythese adsorbents to the maximum extent which is also quite evidentfromthepreviousresults.
The following conclusions were made:
3. Babel,S.Kurniawan,TA,2003.Low-costadsorbents forheavymetalsuptakefromcontaminatedwater: areview.J.HazardousMater.897,219-243
Astheadsorbentdosageincreases,dyeremovalefficiency alsoincreases.Thismaybeduetothegreateravailabilityof adsorptionsites.Nosignificantincreaseinthedyeremoval wasobservedbeyondtheoptimumvalues.
Asthecontacttimeincreases,dyeremovalefficiencyalso increases.Thismaybeduetothegreateravailabilityoftime fortheadsorbentstoadsorbthedyespresentinthewaste water.
Higherdyeremovalwasobservedathigherbeddepth. Thismaybeduetolargesurfaceareaoftheadsorbentand more contact time with the adsorbent to interact with adsorbate.
Thewaterthatwastreatedappearstobecolorlesssothat itcanbeusedforredyeingprocess.
Redyed sample using treated water shows better dye uptakewhichwasequivalenttooriginalsampleasobserved fromK/Svalue
. Finally it may be concluded that the normal adsorbents have profound effect on colour removal efficiency from textiledyewastewater.Thetreatedwatercouldbeusedfor redyeingpurpose
Sametypeofexperimentprocedurecanbecarriedouton textilewastewater
Similar experiments can be carried out with varying quantitiesofsugarcanebagassepowderasabsorbent.
4. Bailey,S.E.,Olin,TJ,Bricka,M.,Adrian,D.D.,1999.A review of potentially lowcost sorbents for heavy metals.WaterRes.33,2469-2479
5. Babu,Parande,S.RaghuandT.Kumar,cottontextile processing: waste generation and effluent treatment.J.CottonSci.11(2007)
6. Banat, P. Nigam, D. Singh and R. Marchant, Microbial decolorization of textile-dye containing effluents.Areview58(1996)217-227.
7. Clare,R.AnlikerIntheHandbookofEnvironmental Chemistry, Part A. Anthropogenic compounds, Hutzinger,Springer,Heidiberg,1980,pp.181-185.
8. Deshkar, S Bokade, S. Dara, Modified Hardwickia binata bark for adsorption of mercury (II) from water,WaterRes.24(1990)1011-1016.
9. Delval,F.,Crini,G.,Vebrel,J.,Knorr,M.,Sauvin,G., Conte,E.2003,Starchmodifiedfiltersusedforthe removalofdyesfromwastewater.Macromol.Symp. 203,165-171.43
10. Environmental Information System Central PollutionControlBoard,MinistryofEnvironment& Forests,Govt.ofIndia,Annualreport1999-2000.
11. EmmanuelOlajideOyeludeandFelixAppiah-Takyi (2011)Removal ofMethyleneBluefromAqueous Solution using Alkali Modified Malted Sorghum Mash, Turkish Journal of Engineering EnvironmentalScience,Vol.36,pp.161-169
Inordertoknowthe economyof processregeneration studiescanhedone.
1. AbmelAdnanAishan(2014)AdsorptionofMethyl Green Dye Bamboo in Barch and Continuous System; Iraqi Journal of Chemical and Petroleum Engineering.Vol15,No1,pp.65-72
2. AzamTMohdDinandBassimHHameed((2010) AdsorptionofMethylVioletDyeonAcidModified ActivatedCarbonIsothermsandthermodynamics, Journal of Applied Sciences in Environmental Sanitation,Vol.5,No.2,pp.161.170
12. ManaseAuta(2012)FixedBedAdsorptionStudies of Rhodamine B Dye Using Oil Palm Empty Fruits Bunch Activated Carbon, Jornal of Engineering ResearchandStudies,Vol.3.pp.03-06
13. Mane, R. S. and Bhusari, V. N. (2004) Removal of Colour(Dyes)FromTextileEffluentbyAdsorption usingOrangeandBananaPeel,InternationalJournal of Engineering Research and Applications, Vol. 2, No.3,pp.1997-200444
14. MohammadArifurRahman,RuhulAmin,S.M.and ShafiqulAlam,A.M.(2012)RemovalofMethylene Blue from Wastewater using Activated Carbon PreparedfromRiceHusk,DhakaUniversityJournal Science,Vol.2,pp.185-189.
15. Nader Yousefi, Ali Fatehizadeh, Elham Azizi, MohammadAhmadian,AbdolkarimAhmadi,Ahmad
Factor value: 7.529 | ISO 9001:2008 Certified Journal |
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056
Volume: 09 Issue: 10 | Oct 2022 www.irjet.net p-ISSN: 2395-0072
Rajabizadeh and Ali Toolabi (2011) Adsorptionof ReactiveBlack 5DyeontoModifiedWheatStraw: Isotherm andKinetics Study, Sacha Journal of Environmental Studies, Vol. 1, No. 2, pp.81-91.p. 136-144.
16. Ozucar and Sengil, 2005; Garg et al., 2004a,b, Baouabetal.,2001;HoandMcKay,1998.
17. Pandey, M. Chaudhuri, Removal of inorganic mercuryfromwaterbybituminouscoal,WaterRes. 16(1982)1113-1118
18. Hameed, B.H., Mahmoud, D.K. and Ahmad, A.L. (2008)EquilibriumModelingandKineticsStudies on the Adsorption of Basic Dye by a Low-Cost Adsorbent:Coconut(CocosNucifera)BunchWaste, JournalofHazardousMaterials,Vol.158,pp.65-72.
2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified
