Investigation the Stability of the Copper Oxide- Ethylene Glycol Nanofluids.
Kusammanavar Basavaraj1*, K Elangovan2 , Mallikarjuna Y1, Veerabhadrappa Algur1 , Purushotham C1, Sagar H S1, Sagiraj Nagaraj1 .2Department of Mechanical Engineering, Er.Perumal Manimekalai College of Engineering, Hosur, India. ***
Abstract - Nano fluid is the suspension of nano sized particles in the base fluid. Nanofluids are tremendous heat transfer applications in the field of thermal engineering such as radiator heat exchanger and solar applications etc. the applications of the CuO-Ethylene glycol (EG) nanofluid in a heat transfer area is essential and maintain the stability of CuO- EG nanofluid is necessary. In the present study two step method was used to prepare the CuO- EG nanofluid without adding surfactant.The sedimentation method was adopted to check the stability of the nanofluid for the volumetric concentration of 0.2%, 0.4%,and 0.6% of CuOnanoparticlesin the EG. The thermo physical properties of the CuO- EG nanofluid was also studied using the appropriate model inthe present work..
Key Words: CuO-EG nanofluid, stability, sedimentation, thermophysicalproperties
1. INTRODUCTION.
Nanofluidsaremostimportantandinnovativefluidsinthe heat transfer applications due to the higher thermal conductivitythenconventionalfluidssuchaswater,ethylene glycol,biofuels,andotheroilswhichareusedtotransferthe heatfromonefluidtoanotherfluids.Inordertoemployee the nano fluids for the heat transfer applications, it is essentialtostudythethermophysicalpropertiesoftheCuOEG nano fluids. The preparation method, stability of the nanofluidsandheattransfercharacteristicsoftheCuO-EG nanofluidsarealsoessentialtofocus.
Amrut. S. et al [1] discussed Synthesis and optical characterizationofcopperoxidenanoparticlesAuthorshoes TEM images,forsize, temimagestoshowthe rectangular morphology of the CuO nanoparticles. Xray diffraction pattern (XRD) reveals single phase monoclinic structure.Authorsalsodescribetheopticalcharacteristicsof theCuOnanoparticles.
Q.Zhang.etal.[2]studiedCharacterizationofnanoparticles andCuOnanostructures:synthesis,characterization,growth mechanisms,fundamentalproperties,andapplications.The authorstellsthecharacteristicsofthenanoparticles.
X.Wang.etal.[3]donetheresearchtomeasuretheThermal conductivityofthenanofluidsforthedifferentconcentration nanoparticles using the hot wire method. But the authors does not use the sedimentation method to measure the stabilityofthenanofluidsfortheheattransferapplications.

The Growth cycle of copper oxide which describes the Copper oxide thin films grown by plasma evaporation methodwasstudiedbyK.Santra.etal.[4].Intheirstudythey Explain about growth of copper oxide nanofluids which helpstodeterminethestabilityofCuOnanofluidfortheheat transferapplications.
A.Aslani.etal.[5]discusstheControllingsystemofcopper oxide nanostructure which explains Controlling the morphologyandsizeofCuOnanostructureswithsynthesis bysolvo/hydrothermalmethodwithoutanyadditives.
Astheliteraturesurveysuggestthatthepreparationofthe CuOEGnanofluidforthevolumetricconcentrationof0.2%, 0.4%,and0.6%usingthetwo-stepmethodwasnotavailable for the 20-nanometre size spherical shape CuO nanoparticles. The sedimentation method was adopted to check the stability of the CuO - EG nano fluid was scant. Hence in the present work the preparation of nano fluid usingthetwo-stepmethodforthedifferentconcentrationof the CuO nanoparticles considered and sedimentation method for stability checking was employed. Apart from these thermo physical properties of the nanofluid such as density,specificheat,thermalconductivityandviscosityfor thedifferentconcentrationofCuOnanoparticlesinEGwere studiedanddiscussed.
2. MATERIALS AND METHODS
TheCuOselectedasnanoparticles andEGasbasefluids were selected based on the literature reviews and gap identified. The size of the CuO nano pparticles is 20 nano meterandinsphericalinshapethemethodologyfollowedfor thepreparationandstabilitycheckingofthenanofluidshown intheFigure1.
2.1 Over view of EG
EG is an organic compound with formula (C2H2OH), it’s mainly used as antifreeze formulations. It is an odorless,
colorless,flammable,viscousliquid.EGhasasweettaste,but itistoxicinhighconcentrations.ItcanoccuratneutralpH andmeltingpointandboilingpointoftheEGare-12.9oCand 198oC respectively. Due its low melting point (freezing) ethyleneglycolusedinmanyheattransferapplications.The thermophysicalpropertiesofdistilledwaterwerementioned intheTable1.
temperature. They are graded harmful to humans and as dangerous for the environment with adverse effect on aquatic life. Chemical composition of copper oxide having copper79.87%andoxygen20.10%.themeltingpointand boiling point of copper oxide are 1201oC and 2000oC respectively. The density and specific heat of the copper oxide nanoparticles is 6315 kg/m3 and 540 J/kg K. the thermalconductivityofthesphericalshaped20nanometer sizedCuOnanoparticlesis32.9W/mKwhichisgivenbythe supplierNanoResearchlab Jamshedpur,India.
2.3 TEM images of CuO nanoparticles
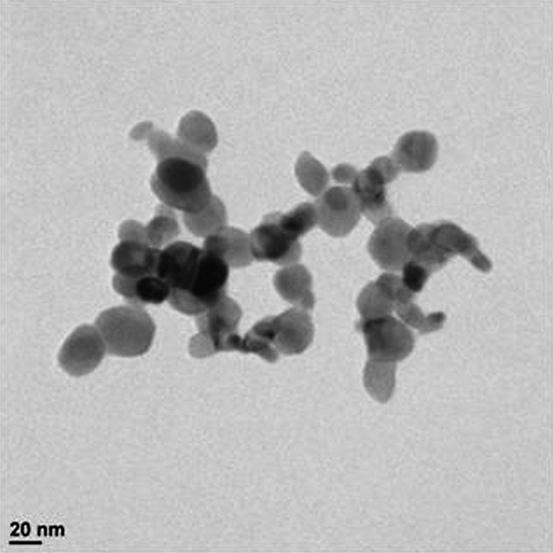
CuO nanopowder was purchased from Nano research lab Jamshedpur,India Company.Themanufacturerconfirmed thatparticlesizewaslessthan50nmandsurfaceareawas 29m2/g. TheCuOparticles wereplacedinEG (10mg/L) and subjected to sonification for 5 minutes to reduce agglomeration before characterization. Particle size was characterizedusingtransmissionelectronmicroscopy(TEM) andsedimentationmethodandparticlesizeanalyzers.TEM wasdoneonaJEM2100F(JEOLLtd.,Japan)operatingat100 kV. The sample for TEM observation was prepared by dispersing the powder of CuO nanoparticles by ultra sonificationinEGandallowingthedispersiontodropona coppergrid.ArepresentativeTEMimageofCuOaggregates isshowninFigure2.TheparticlesizesofCuOnanoparticles weremeasuredwithanELS-6000analyzer(PhotalOtsuka Electronics,Japan)andshowninnm.
2.4 X-RD images of CuO nanoparticles
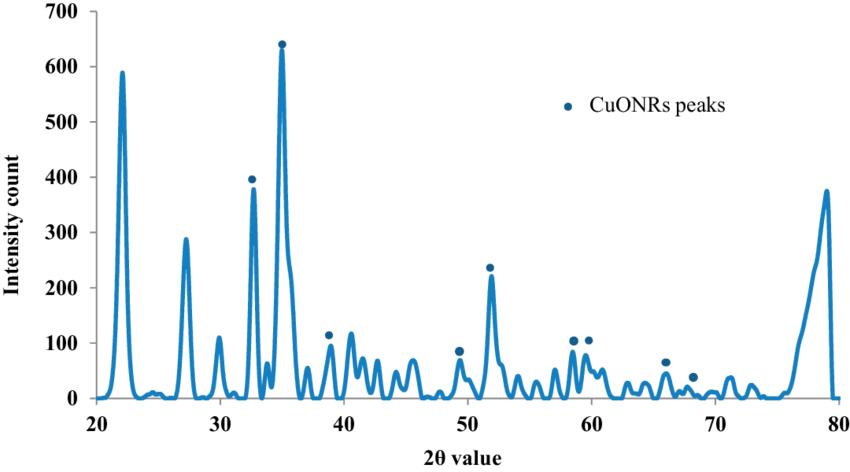
ThemicrocrystallinestructureofCuONRwasanalyzedusing theXRDtechnique.ThegraphwaspreparedusingPowderX software.AsshowninFigure3,thecharacteristicXRDpeaks wereobservedat32.64,35.1,38.9,48.9,52.0,58.46,62.9, 65.94and67.96correspondingto110,002,111,202,020, 202, 113, 311 and 113 reflections respectively which indicate the formation of typical monoclinic CuO NR structure and are in agreement with the standard values reportedbytheJCPDScardno.801268andICDDcardno. 801916 which was in accordance with previous studies reported. However, other peaks are also denoted in the figure.Theaveragecrystallitesizewascalculatedtobe20 nmusingDebyeScherrer’sequation
D=Kλ/(βcosθ)
Fig -1:Methodology

2.2 Over view of copper oxide (CuO) nano particles
Copperoxidenanoparticlesappearasabrownish-black powder. They can be reduced to metallic copper when exposed to hydrogen or carbon monoxide under high
WhereDisanaverageparticlesize(nm),Kistheconstant andequalsto0.94,λisthewavelengthofX-rayradiation,βis full-widthathalfmaximum(FWHM)ofthepeakinradians andθisthediffractionangle(degree).
2.6 Preparation of the nano fluids
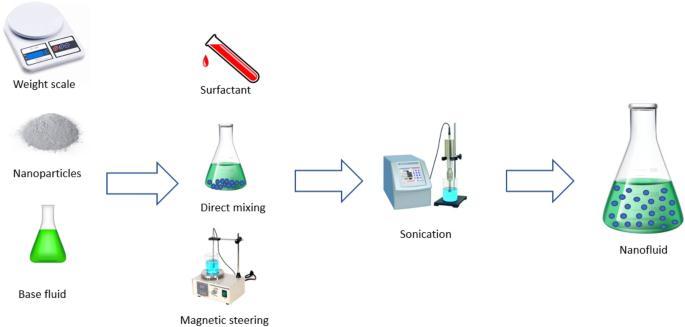
CuO–EGnanofluidwaspreparedwithverylowconcentration of surfactant-less. In this work CuO nanoparticles with averagediameterof20nmweredispersedinEGnanofluid usingmagnetic stirrerwithhotplateat700rpmand35oC temperature (Make: SESW). For each volume fraction requiredCuOnanopowderwasaddedtoEGandexposedto shear homogenization for 20 min at 700 rpm speeds and followedbyhigherspeeds.Two-steppreparationprocessis extensivelyusedinthesynthesisofnanofluidsbymixingbase fluids with commercially available nanopowders obtained fromdifferentmechanical,physicalandchemicalroutessuch as milling, grinding,and sol-gel and vapor phase methods. ThetwostepmethodofpreparationshowninFigure4.
2.7 Sedimentation method forstability checking of nanofluids
Stabilityisabigissuethatinherentlyrelatedtothisoperation asthepowderseasilyaggregateduetostrongvanderWalls forceamongnanoparticles.Stabilityofnanofluidisimportant to get the same thermophysical properties. Stability of nanofluidisrelatedtoelectricaldoublelayerrepulsiveforce
andVanderWaalsattractiveforce.ElectricalDoubleLayer Repulsive Force (EDLRF) must be higher than the Vander Waalsattractiveforcestogetstablenanofluid.VanderWaals attractive forces between nanoparticles causes to get clustered because of attraction forces. If this force is high, nanoparticles get separated from base fluid and these clusterednanoparticlessettledownatthebottomofvessel becauseofgravitationalforce.Ontheotherhand,EDLRFacts asoppositetoVanderWaalsattractiveforcewhichseparates the particles from each other. The sedimentation was adoptedforthestabilitycheckingofthenanofluidwhichis basicmethodandrequirelongerperiod.
2.8 Thermo physical properties of the CuO-EG nanofluids.
Density of the nanofluid
Densityismassperunitvolume,PakandChaodevelopedthe correlationstocalculatethedensityofthenanofluidbytaking theaccountofdensityofnanoparticlesandbasefluids.
ρnf=Φρp+(1-Φ)ρf (1) Specific heat

Specificheatisthecapacityofthenanofluid.Specificheatis the depends on the density, volume concentrations, and specificheatofthenanoparticlesandbasefluid.
(2)
Thermal Conductivity
Thermal conductivity is the property of the materials and function of temperature. Nanofluid found in many heat transfer applications. The thermal conductivity of the nanofluid calculated using the Maxwell correlations of equation.
Viscosity
The Einstein developed the correlations to calculate the viscosityofnanofluid.InthepresentworkviscosityofCuOEG nanofluidcalculatedusingtheEinsteinmodel.
2.9 Volumetric concentrations to gravimetric
concentrations.
The volumetric concentration of the CuO nanoparticles convertedtogravimetric(mass)withthefollowingequation. (5)
3. RESULTS AND DISCUSSIONS
The research was carried out to determine the thermo physicalpropertiesandstabilityofthe20nmsizedspherical shapedCuO–EG nanofluidfortheconcentrationsof0.2%, 0.4%, and 0.6% of CuO nanoparticles without adding surfactant.Theresultswerediscussedasfollows.

3.1 Thermo physical properties.
Thethermophysical propertiesoftheCuO –EG nanofluids such as density, specific heat, thermal conductivity and viscositywerecalculatedanddiscussed.
Density of the CuO-EG nanofluids
ThevariationofdensityoftheCuO–EGnanofluidatdifferent concentrationshowninFigure5.ThedensiyoftheCuO–EG nanofluid incresed with incresed concentrations. The concentration of tha CuO – EG nanofluid vary from 0.2%, 0.4%, and 0.6%. The valeus of density at 0.2%, 0.4%, and 0.6% were 1124.80 kg/m3, 1135.20 kg/m3 and 1145.60 kg/m3respectively.
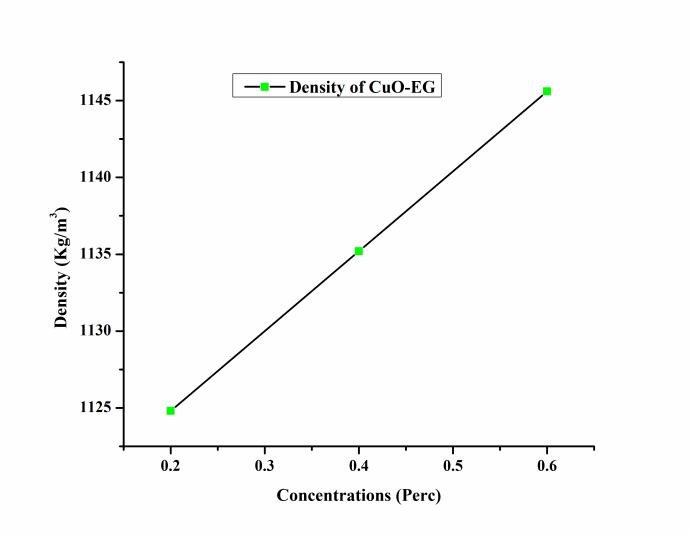
The maximum density occurred at 0.6% and minimum at 0.2% shown in Figure 5. Due to increase in the density viscosityincreasesandclusteredofnanoparticlesintheEG increased.Thedensityofthenanofluidwasmeasuredwith relations(1).
Thermal Conductivity of the CuO-EG nanofluids
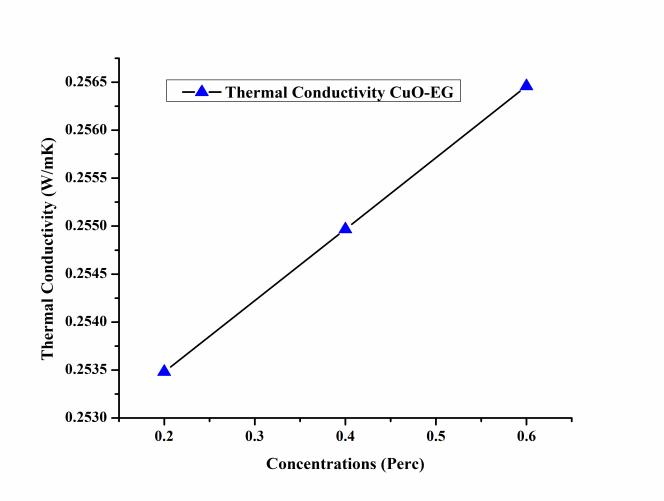
The variation of thermal conductivity of the CuO – EG nanofluidatdifferentconcentrationshowninFigure6.The thermalconductivity oftheCuO–EGnanofluidincresedwith incresedconcentrations.TheconcentrationofthaCuO–EG nanofluid vary from 0.2%, 0.4%, and 0.6%. The values of thermalconductivityat0.2%,0.4%,and0.6%were0.2534 W/mK,0.2549W/mKand0.25645W/mKrespectively.The maximum thermal conductivity occurred at 0.6% and minimumat0.2%showninFigure6.Duetoincreaseinthe thermal conductivity, heat transfer through nanofluid increasedcomparedtoEG.Thethermalconductivityofthe nanofluidwasmeasuredwithrelations(3).
Viscosity of the CuO – EG nanofluids
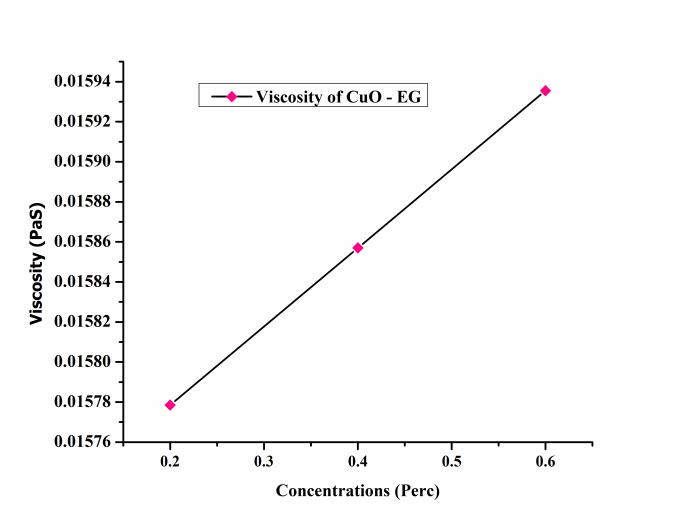
The variation of viscosity of the CuO – EG nanofluid at differentconcentrationshowninFigure7.Theviscosity of the CuO – EG nanofluid incresed with incresed concentrations.TheconcentrationofthaCuO–EGnanofluid varyfrom0.2%,0.4%,and0.6%.Thevaluesofviscosityat 0.2%, 0.4%, and 0.6% were 0.0157 paS, 0.0158 paS and 0.0159paSrespectively.Themaximumviscosityoccurredat 0.6%andminimumat0.2%showninfigure.Duetoincrease in the viscosity, heat transfer through nanofluid increased comparedtoEG.Theviscosityofthenanofluidwasmeasured withrelations(4).
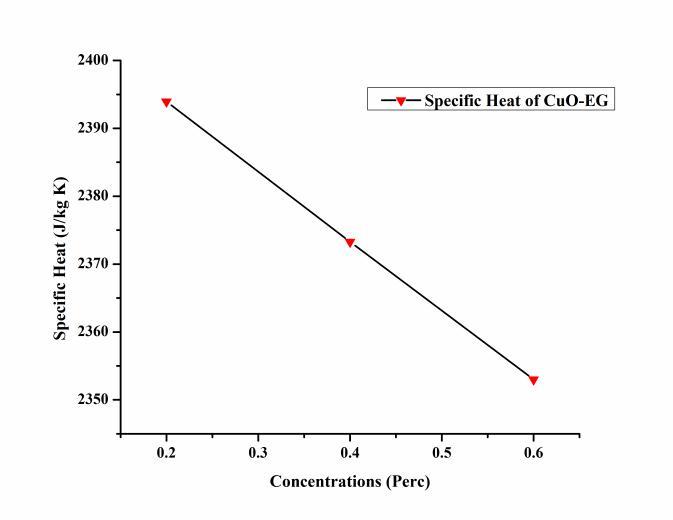
0.4%,and0.6%.Thevaluesofspecificheatat0.2%,0.4%,and 0.6%were2393.94J/kgK,2373.27J/kgKand2352.98J/kg Krespectively.Themaximumspecificheatoccurredat0.2% andminimumat0.6%showninfigure.Duetodecreaseinthe specific heat, heat transfer through nanofluid increased compared to EG. The specific heat of the nanofluid was measuredwithrelations(2).

3.2 Preparation of the nanofluid.
Table -2: ThemassoftheCuOnanoparticleinEG
the
The Specific Heat of the CuO – EG nanofluids
differentconcentration
The specific heat of the CuO – EG nanofluid at different concentrationshowninFigure8.Thespecificheat oftheCuO
EG nanofluid incresed with incresedconcentrations.The concentration of tha CuO – EG nanofluid vary from 0.2%,
The nanofluid prepared using two step methods without additionofsurfactant.The20nmsizedsphericalshapedCuO nanoparticleswithconcentrationsof0.2%,0.4%,and0.6% wereconvertedtomassusingequation(5)andtabulatedin theTable2,mixedwith20mlofEGandstirredwithmagnetic stirrerwithhotplateat700rpmand35oCfor20mintoavoid theclusteringofthenanoparticlesanduniformdistribution


ofthenanoparticlesintheEG.Thesampleswereshownin Figure 9 & 10. The prepared nanofluidskept for 5 days to studythesedimentationofthenanoparticles.
3.3 Stability of the CuO – EG nanofluids
The sedimentation method was adapted to determination Stability of the CuO - DI water nanofluids. The Prepared samples of the nanofluids kept for 5 days to monitor the settlementofthenanoparticlesintheEG.TheDigitalcamera used to take the photos of the samples on daily basis to observeanddeterminethesettlementofthenanoparticlesin theEG.Thenanofluidwhichtookmoretimetosettledownis saidtobemorestablenanofluidusedfortheheattransfer applications.ThestabilityresultsoftheCuO–EGnanofluidat differentconcentrationshowninTable3.
Thestabilityofthenanofluidsmoreatlowconcentrationi,e at 0.2% and decreases as the concentrations increases to 0.4%and0.6%duetoincreaseinthedensityandviscosityof the CuO nanoparticles in the EG. Hence the higher concentrations are having less stable compared to lower concentrations.
Table – 3: ThestabilityoftheCuO–DIwaterNanofluids atdifferentconcentrations
Φ ConcentrationsPercentage.
Subscript
np nanopartlces
bf Basefluid
nf Nanofluid
Abbreviations:
EG Ethyleneglycol
NP nanoparticles
ACKNOWLEDGEMENT
All the authors are contributed and no funding for the researchwork.
REFERENCES
[1]. Amrut.S.Lanje,SatishJ.Sharma,RamchandaraB. Pode , Raghumani S. Ningthoujam, 2010, “Synthesis and optical characterization of copper oxide nanoparticles”, AdvancesinAppliedScience Research,2010,1(2):36-40, ISSN:0976-8610.
[2]. QiaobaoZhang, KailiZhang, DaguoXu,2013,“CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications”, Progress in Materials Science 60(1):208–337 DOI:10.1016/j.pmatsci.2013.09.003.
[3]. Wang, X., Xu, X. and S. Choi, S. U. 1999. “Thermal conductivity of nanoparticle-fluid mixture”, Journal of ThermophysicsandHeatTransfer,13:474-480.
3. CONCLUSIONS
1. Thedensity,viscosityandthermal conductivityof the CuO-EG nanofluids increased with increase in concentrationsofthenanoparticles.
2. Thespecificheatofthenanofluidsdecreaseswith increasedconcentrationsoftheCuOnanoparticlesintheEG.
3. Thetwostepmethodwaseconomicalandsuitable methodforthepreparationofthenanofluids.
4. TheCuO–EGnanofluidwasstableat0.2%volume concentrations of CuO nanoparticles. The stability of the CuO-EG nanofluid decreases with increases in the concentrations.
Nomeclature:
ρ Densitykg/m3
Cp SpecificheatJ/kgK
µ DynamicviscosityPaS
K ThermalConductivityW/mK.
[4]. Apurba Kumar Santra, Swarnendu Sen, Niladri Chakraborty,“Studyofheattransferduetolaminarflowof copper–water nanofluid through two isothermally heated parallel plates”, International Journal of Thermal Sciences,Volume 48, Issue 2, 2009, Pages 391-400, ISSN 1290-0729, https://doi.org/10.1016/j.ijthermalsci.2008.10.004.

[5]. Alireza Aslani Zakariya, “Controlling the morphologyandsizeofCuOnanostructureswithsynthesis by solvo/hydrothermal method without any additives”, January2011,PhysicaBCondensedMatter406(2):150-154, DOI:10.1016/j.physb.2010.10.017.
[6]. Ali, M. and Zeitoun, O. 2009. Nanofluids forced convectionheattransferinsidecirculartubes.International JournalofNanoparticles,2:164-172.
[7]. Bahiraei, M., Hosseinalipour, S. M., Zabihi, K. and Taheran,E.2012.Usingneuralnetworkfordeterminationof viscosityinwater-TiO2nanofluid.AdvancesinMechanical Engineering,2012:1687-8132
[8]. Beck,M.P.,Yuan,Y.,Warrier,P.andTeja,A.S.2009. The effect of particle size on the thermal conductivity of alumina nanofluids. Journal of Nanoparticle Research, 11: 1129-1136.
[9]. Bobbo,S.,Fedele,L.,Benetti,A.,Colla,L.,Fabrizio,M., Pagura, C. and Barison, S. 2012. Viscosity of water based SWCNH and TiO2 nanofluids. Experimental Thermal and FluidScience,36:65-71.
[10]. Choi,S.U.andEastman,J.1995.Enhancingthermal conductivityoffluidswithnanoparticles.Developmentsand Applications of Non-Newtonian Flows, FED 231/MD 66, ASME:99-105.
[11]. Das, S.K., Choi, S.U., Yu, W. and Pradeep, T. 2007. Nanofluids: science and technology, Wiley-Interscience Hoboken,NJ.
[12]. Das, S.K. 2008. Monitoring dopants by Raman scattering in an electrochemically topgated graphene transistor.Naturenanotechnology,3.4:210-215.
[13]. Duangthongsuk,W.andWongwises,S.2009.Heat transferenhancementandpressuredropcharacteristicsof TiO2–water nanofluid in a double-tube counter flow heat exchanger.InternationalJournalofHeatandMassTransfer, 52:2059-2067.
[14]. Fedele,L.,Colla,L.andBobbo,S.2012.Viscosityand thermal conductivity measurements of water-based nanofluids containing titanium oxide nanoparticles. InternationalJournalofRefrigeration,35:1359-1366.
[15]. Gosselin,L.anddaSilva,A.K.2004.Combined“heat transfer and power dissipation” optimization of nanofluid flows.AppliedPhysicsLetters,85:4160-4162.
[16]. Han, W.S. and Rhi, S.H. 2011. Thermal characteristicsofgroovedheatpipewithhybridnanofluids. ThermalScience,15,195-206.
BIOGRAPHIES
Prof.KusammanavarBasavaraj
Dept:MechanicalEngineering
RYMEC,Ballari,India
Dr.ElangovanK.

Dept:MechanicalEngineering
Er.Perumal Manimekalai College of Engineering, Hosur, India.
Prof MallikarjunaY
Dept:MechanicalEngineering
RYMEC,Ballari,India
Dr.VeerabhadrappaAlgur
Dept:MechanicalEngineering
RYMEC,Ballari,India
Mr.PurushothamC






Dept:MechanicalEngineering
RYMEC,Ballari,India
UGstudent
Mr.SagirajNagaraj
Dept:MechanicalEngineering
RYMEC,Ballari,India
UGstudent
Mr.SagarHS
Dept:MechanicalEngineering

RYMEC,Ballari,India
UGstudent
