Removal of Fluoride From Drinking Water Using Tea Waste as Adsorbent
Aparna K T1 , Varsha Ashokan21M.Tech scholar in Environmental Engineering, Department of Civil Engineering, M. Dasan Institute of Technology, Kozhikode, Kerala, India

2Assistant Professor, Department of Civil Engineering, M. Dasan Institute of Technology, Kozhikode, Kerala, India ***
Abstract - Fluoride concentration above 1.5 ppm in drinking water can cause serious health hazards. A low cost and highly efficient adsorbent is prepared by using tea waste and ability to remove fluoride from drinking water is tested. Tea waste is cleaned and chemically treated with sulphuric acid and formaldehyde which is then digested in alum. The fluoride removal studies are done by adsorption method by tea waste. The effect of contact time, pH and adsorbent dosage are considered in batch study. For column study, a column with 50 mm diameter and height 60cm is used. The experiment is conducted for varying bed depth of 3.5 cm, 5.4 cm, 8.9 cm and flow rate of 5ml/min and 10ml/min. Regeneration study is conducted by using sodium hydroxid and desorption efficiency of cycle 1 and cycle 2 is compared. The exhausted adsorbent is regenerated upto two cycles.
Key Words: Fluoride, Adsorption, regeneration, column, batch, removal.
1. INTRODUCTION
Groundwaterisarenewableresource,yettheworld’ssupply ofgroundwaterissteadilydecreasingespeciallyinAsiaand NorthAmerica[3].Groundwaterqualitydeteriorationand supplyofsafedrinkingwaterisamajorconcernthroughout the world. Groundwater with high fluoride concentration (>1.5 mg/L), according to WHO (2011), is affecting more than260millionpeoplearoundtheworld[1].
Groundwater is one of the primary sources of water for domesticandagricultureusesinKerala.Duringthelasttwo decades, the groundwater scenario of Kerala has been witnessingseveralchanges[2].
2. MATERIALS AND METHODOLOGY
2.1 Adsorbent Used
Camellia sinensis is a species of evergreen shrubs from which an aromatic bevarage called tea is made. It is originatedinborderlandsofChinaandNorthernMyanmar. Recentlytheteaconsumptionaroundtheworldisincreasing whichresultsin theproduction of tea leafwastefrom the industrialscaleextractionoftealeavestoproduceinstant teaandbottledteadrinks.
2.2 Adsorbent Preparation
Inthisstudywasteteadustfromthehomeisusedtocarry out the experiment. The waste tea dust is collected and washedproperlytoremovethemilkandsugar.Thenboiled twice inorder to remove the colour. The tea waste is sun driedtoremovemoisture.Thedriedteawasteissievedto gettheparticleshavingsize250µm-500µm.Theoversized particles are grounded by mortar and pestle and again sieved.
Forchemicaltreatmenttake10goftealeavesandadd100 mL of 0.4 N H2SO4 and 20 ml of 30% formaldehyde. This mixture is kept at a constant temperature of 50° C for 3 hours. Then tea leaves washed with distilled water to removetheacidandformaldehyde.Thenitiskeptinhotair oventoremovemoisture.Tealeavesisthendigestedin2% alum solution. This is designated as tea leaves chemically treatedwithsulphuricacidandformaldehyde.
2.3 Collection of Sample
ThesamplewascollectedfromPalakkaddistrictandvarious parameters such as pH, TDS, Total hardness, Electrical conductivity,AlkalinityandFluoridearetested.
2.4 Preparation of Fluoride Solution
The required sample solution is made up by diluting Fluoride standard solution traceable to SRM (Standard Reference Material) from NIST (National Institute of StandardsandTechnology)NaFinH2O,madeinGermany, EMDMilliporeCorporation.
2.5 SPADNS Spectrophotometric Method
SPADSNSspectrophotometricmethodisacommonlyused methodforthedeterminationoffluorideindrinkingwater.
This method involves the reaction of fluoride with a red zirconium - dye solution. The basic principle of spectrophotometricmethodisthateachcompoundabsorbs ortransmitslightoveracertainrangeofwavelength.
2.6 Batch Study
Batch study is conducted in order to study the effects of variousparameterslikeadsorbentsize,adsorbentdosage, Initialfluorideconcentration,pHandcontacttime.
Andadsorbentdosage3g/l,5g/l,7g/l,9g/l,11g/l,13g/l, 15g/l, were considered. Initial fluoride concentrations consideredwere1mg/l,3mg/l,5mg/land7mg/l.Contact timeconsideredwere30,60,90,120,150and180minutes.
2.7 Column Study
Inordertoinvestigatethepracticalaspectofapplicationof tea leaves for the removal of fluoride column study is conducted by considering bed depth and flow rate as parameters.ThecolumnexperimentiscarriedoutinaPVC pipeof50mmdiameterand60cmlengthanditisattached to a flow control valve for adjusting the flow through column. Bed depth of 3.5 cm, 5.4 cm and 8.9 cm are considered.Andflowrateof5ml/minand10ml/minare considered.
2.8 Regeneration of Adsorbent
In the environmental aspect and economic aspect the regenerationofadsorbentisimportant.Hereregeneration studyisconductedusingSodiumHydroxideasregenerant. After saturation of adsorbent the regenerant is passed throughtheadsorbentbedandfluorideremovalischecked. The experimental setup used for regeneration was same whichwasusedforthebreakpointanalysis.Thebeddepth ofadsorbentusedwas5.4cmataflowrateof5ml/minand initialfluorideconcentrationof3mg/l.
3. RESULTS AND DISCUSSION
3.1 Testing of Raw Water
The collected sample of water is tested for various parameters. The values had been compared with the drinkingwaterstandardsIS10500:2012.
Alltheparametersexcepthardnessandfluorideiswithinthe desirable limit as per Drinking water standards IS 10500:2012.Thevalueof fluorideinthesamplecollected from Palakkad district is 2.63 mg/l which is above the desirablelimitof1mg/l.
3.2 BATCH STUDY
In batch study Adsorbent size, Adsorbent dosage, initial fluorideconcentrationpHandcontacttimewereconsidered, the detailed results of these parameters are included in followingsections.
(i) Effect of Adsorbent size
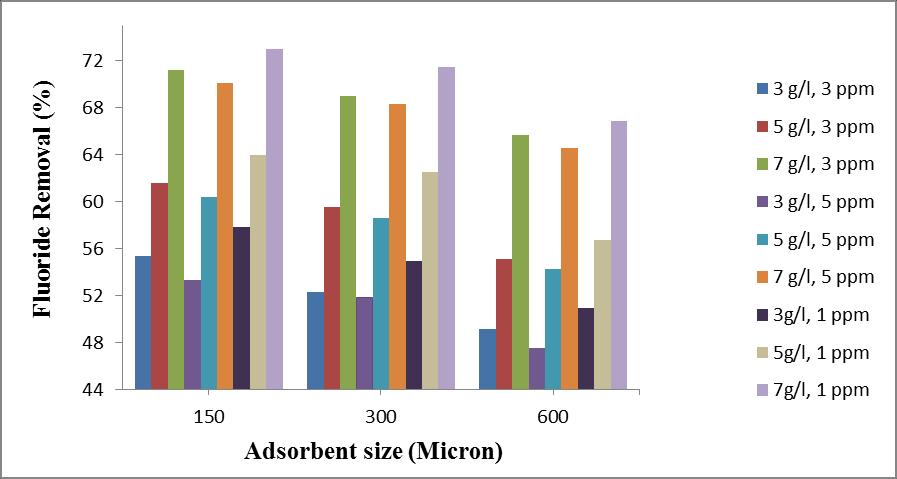
Thestudyoftheeffectofadsorbentsizeonfluorideremoval efficiencywascarriedoutbyusing150µm,300µm,600µm sizedteawaste.
Chart – 1:ComparisonontheeffectofAdsorbentsizeon theremovalefficiencyofFluorideatdifferentinitial fluorideconcentrationsof1mg/l,3mg/land5mg/land adsorbentdosagesof3g/l,5g/land7g/l.
Amaximumof73%removalwasobtainedfor150µmsized particlesofadsorbentatanadsorbentdosageof7g/land initial fluoride concentration of 1 mg/l and 71.5 % is obtained for 300µm sized adsorbent, but considering the 600µmsizedadsorbenttheremovalefficiencydeclinesto 66.90%at3mg/linitialfluorideconcentration.
(ii) Effect of Adsorbent Dosage
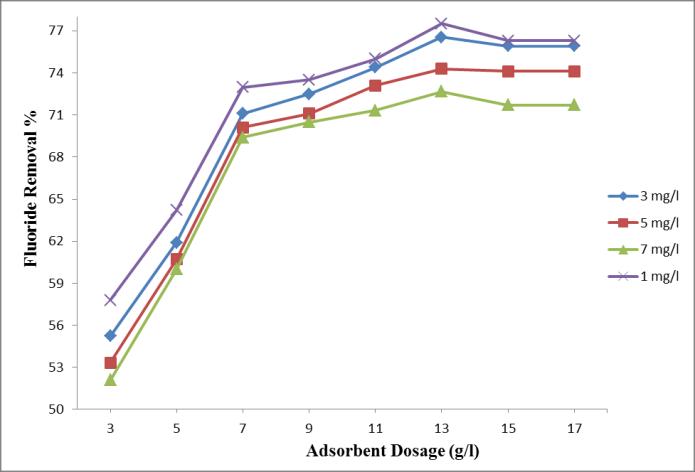

Thestudyoftheeffectofadsorbentdosageonthefluoride removalefficiencywascarriedoutwithadosageof3g/l,5 g/l,7g/l,9g/l,11g/l,13g/l,15g/l.Thestudyisdoneby keeping adsorbent size as 150 µm and varying initial fluorideconcentrationas3mg/l,5mg/land7mg/l.
Chart 2: -EffectofadsorbentdosageonFluorideRemoval atdifferentinitialfluorideconcentrationsof1mg/l,3 mg/l,5mg/land7mg/l.
Amaximumefficiencyof76.54%and74.3%wasobtained at13g/ladsorbentdosage,atinitialfluorideconcentration3 mg/land5mg/l.And72.67%wasobtainedat7mg/linitial fluorideconcentration.
(iii) Effect of Initial Fluoride Concentration and Contact time
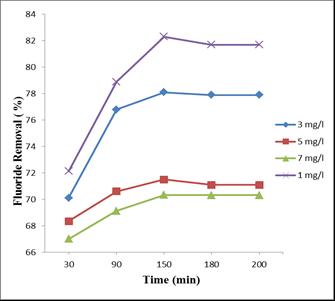
Thestudyisconductedbyvaryingtheconcentrationsfrom1 mg/l, 3 mg/l, 5 mg/l and 7 mg/l, while keeping the adsorbentsizeanddosageas150µmand13g/l.

Themaximumremovalefficiencyisobtainedat1mg/linitial fluoride concentration. We can see that the percentage removal has decreased with the increase in initial concentrationoffluoride.
ThemaximumremovalefficiencyisobtainedatapHof6.5 andthereisnogreatvariationinremovalefficiencywithin pH6.5to8.5.
3.3
Incolumnstudywearemainlyconsideringparameterssuch asbeddepthofadsorbentandflowrate.
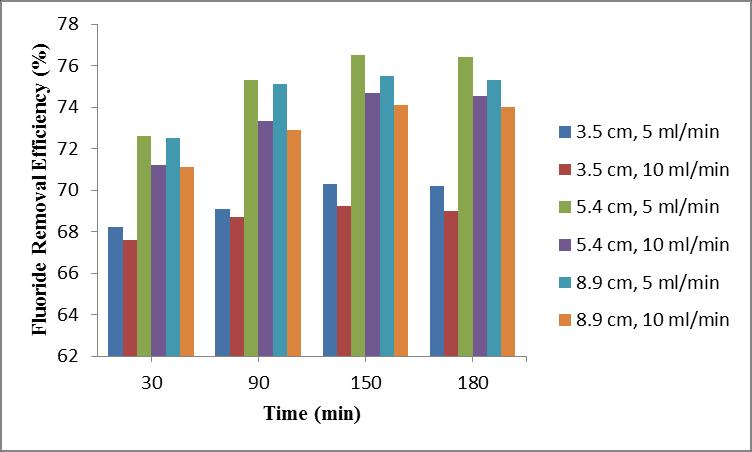
(i) Effect of Bed Depth and Flow Rate
Inthisexperimentthedepthvariesfrom3.5cm,5.4cmand 8.9cmandthedifferentflowratechoosenare5ml/minand 10ml/minattimeintervalof30,90,150and180minutesat pH6.
(iv) Effect of pH
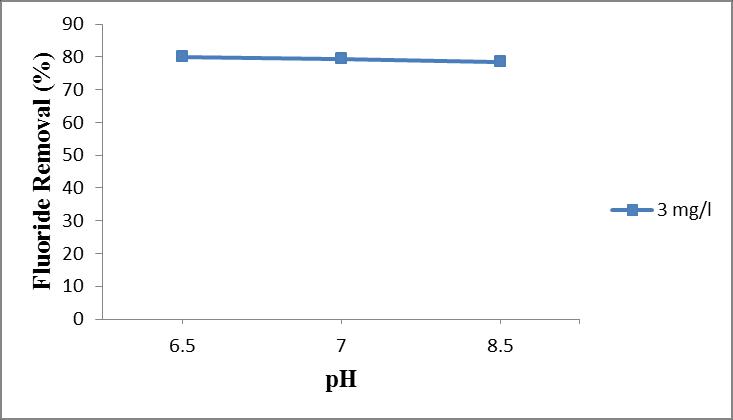
TheeffectofpHontheextentofremovalofthefluoridewas studiedbyvaryingthepHfrom6.5,7and8.5whilekeeping the adsorbent size and dosage as 150 µm and 13 g/l and initialfluorideconcentration3mg/l.
Themaximumremovalefficiencyof76.5%isobtainedata adsorbentbeddepthof5.4cmandataflowrateof5ml/min after150minutes.Butafter180minutesthereisadecrease in the fluoride removal efficiency. Removal efficiency decreasedfrom76.5%to76.41%.
(ii)Break through Study
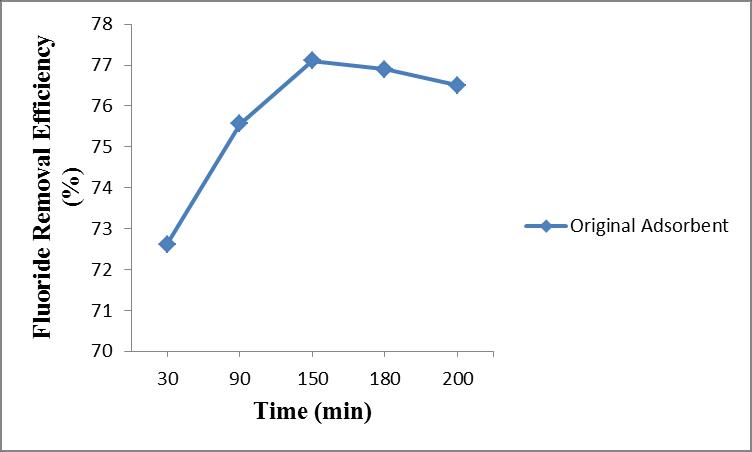
Inorder to find the breakthrough time of column we conductedthestudytillthesaturationofthecolumninthe firstcycle.
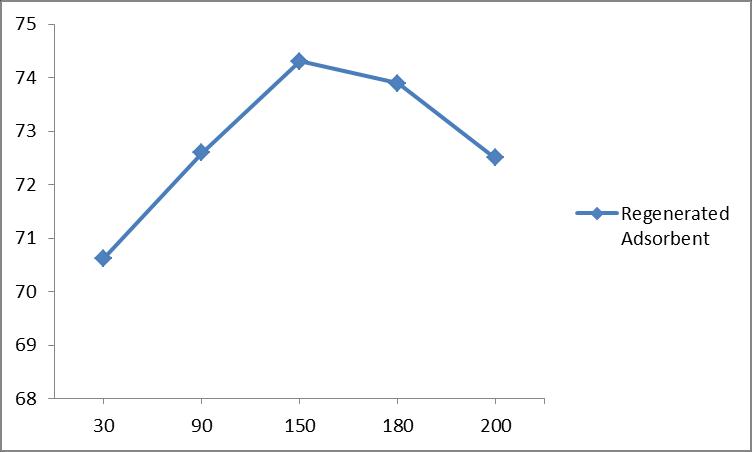
A fluoride removal efficiency of 72.62 % obtained at 30 minutesandgraduallyincreasesto75.56%at90minutes and 77.1%removalat150minutes.
This study shows that the regeneration of adsorbent is possible and a maximum fluoride removal of 74.9 % and 68.13%isobtainedincycle1andcycle2.
4. CONCLUSIONS
3.4 REGENERATION STUDY
We need to check the possibility of regeneration before throwingtheadsorbentafteritreachesthesaturationpoint. SodiumHydroxidesolutionwasusedasregenerant.
After saturationofadsorbentcolumn incycle1 iswashed withNaOH solution.Initiallya fluoride removal of 66.4 % occursat10minutes,thengraduallyincreasesto67.1%in 30minutes,68.6%in50minutes,69.73%in70minutes, 71.33%in90minutes,73.1%in110minutes,74.9%in130 minutes,thenreducedto74.6%in150minutesand72.8% in170minutes.
Incycle2initiallyfluorideremovalefficiencywas60.33%in 10 minutes, then gradually increases to 62.51 % in 30 minutes,63.7%in50minutes,64.11%in70minutes,65.4 % in 90 minutes, 67.5 % in110 minutes, 68.13 % in 130 minutes,thenreducedto67.12%in150minutes,65.3%in 170minutes.
Theadsorbentsizeusedinthisstudyare150µm,300µm and600µm.TheAdsorbentdosageare3g/l,5g/l,7g/l,9 g/l, 11 g/l, 13 g/l and 15 g/l. The initial fluoride concentration of 1mg/l, 3 mg/l, 5 mg/l, 7 mg/l. The time varyingfrom30minutesto180minutesascontacttime.The effectofpHonfluorideremovalefficiencywasconductedby varyingpHfrom6to8.Maxremovalefficiencywasobtained for150µmadsorbentsizeand13g/ladsorbentdosagewith 3mg/linitialfluorideconcentrationat150minutesatpH6. Inthecolumnstudytheparametersmainlyconsideredwere flowrateandBeddepth.Flowratechoosenforthecolumn studywere5ml/minand10ml/min.Thebeddepthchoosen were3.5cm,5.4cmand8.9cm.Asthefirstcyclecompleted a maximum fluoride removal efficiency of 77.1 % was obtainedatabeddepthof5.4cmandflowrateof5ml/min in breakthrough analysis. And as the second cycle has completedamaximumremovalefficiencyof 74.31%was also obtained at a bed depth of 5.4 cm and flow rate of 5 ml/min.
Inregenerationstudyofcycle1thefluorideremovalof66.4 % was obtained at 10 minutes the gradually increased to 74.9%at130minutes.
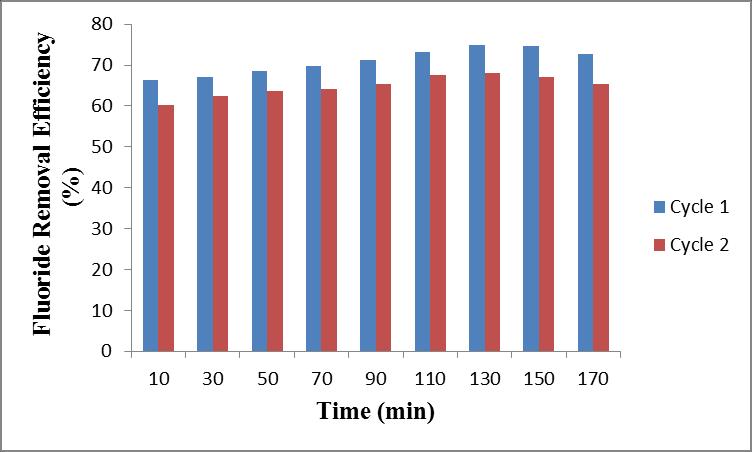
Incycle2thefluorideremovalof60.33%wasobtainedat 10 minutes then gradually increased to 68.13 % at 130 minutes.After130minutestheremovalefficiencyreduced to67.12%at150minutesandthendeclinedto65.3%at 170minutes.
Fromthissetsofexperiment,wecanconcludethatteawaste can be used as an adsorbent for removing fluoride from water,asitshowsgreatremovalefficiencyandregeneration characteristics.

REFERENCES
[1] Amini,M.,Mueller,K.,Abbaspour,K.C.,Rosenberg,T., Afyuni,M.,Moller,K.N.,Sarr,M.,Johnson,C.A.,(2008) Statistical modeling of global geogenic fluoride contaminationingroundwaters,Environmentalscience andtechnology,42,3662-3668.
[2] E.Shaji&Bindu,&Viju,J.&Thambi,D.S.(2007).High fluoride in groundwater of Palghat District, Kerala. CurrentScience.92.240-245.
[3] Gleeson, T., Wada, Y., Bierkens, M. F., Van Beek, L. P., (2012) Water balance of global aquifers revealed by groundwaterfootprint,Nature488(7410),197-200.
