Neutralization of Acidic Effluent from Sugar Mill using Sodium Hydroxide Flakes

Yash Inamke1 and Shruti Gore2
1MTech, VIIT, Pune, Maharashtra, India.
2Asst. Prof., VIIT, Pune, Maharashtra, India. ***

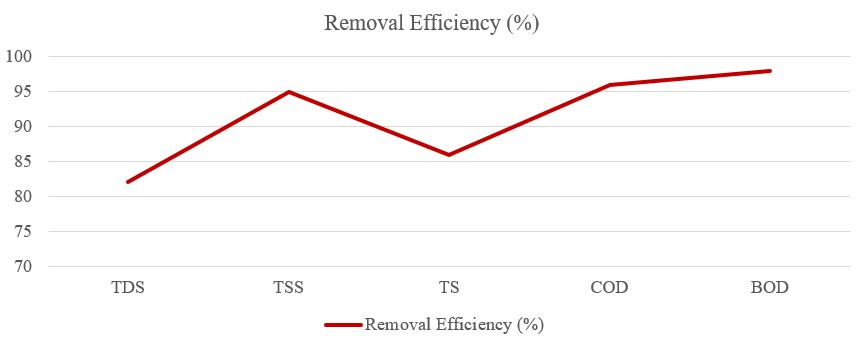
Abstract - The Sugar industry is one of the most polluting industries, not only in terms of the volume of effluent generated but also in terms of its characteristics as well. Most of the sugar industries uses lime as a neutralizerinETP. Inthis experiment, Sodium hydroxide flakes (NaOH) were used to neutralize acidic effluent from the sugar mill. The performance of sodium hydroxide flakes used for effluent neutralization was evaluated. The experiment was performed using a jar test and its effects on parameters such as pH, total dissolved solids, total suspended solids, total solids, Chemical Oxygen Demand (COD), and Biochemical Oxygen Demand (BOD). During the production of sugar, a large amount of water is used by the sugar mill for processing, thus generating a large amount of effluent. The amount required for sodium hydroxide to raise pH to 7.5 was 0.20 mg/L which is two to three times lower than the amount required for conventional alkalis to raise pH. The results of the experiment showthatthe removal efficiency of TDS, TSS, TS, COD, and BOD are 82%, 95%, 86%, 96%, and 98%.
Key Words: Sodiumhydroxideflakes,pH,TDS,TSS,TS,COD, BOD.
1.INTRODUCTION
ThesugarindustryisoneofthemostimportantArgo-based industries in India and has a high impact on the rural economy. India continued to rank second among the countries of the world in sugar production. In India, the sugarindustryrankssecondinArgo-basedindustries.The sugarindustryisseasonalandoperatesfor5-6monthsina year.Theeffluentfromthesugarmillisprimarilygenerated from the mill house, boiler blow down, filter cloth, condensates,condenserswashing,occasionalleakages,and molassesmixedwater.TheSugarindustryisoneofthemost polluting industries, not only in terms of the volume of effluentgeneratedbutalsointermsofitscharacteristicsas well. It generates about 1000 liters of effluent per ton of processed sugar. The volume, concentration, and compositionoftheeffluentsarisinginthesugarindustryare dependent on the type of product being processed, the productionprogram,operatingmethods,thedesignofthe processing plant, the degree of water management being applied,andsubsequentlytheamountofwaterisconserved.
Sugarmilleffluentischaracterizedbyhightemperature, high biochemical oxygen demand (BOD), and chemical
oxygen demand (COD) concentrations, and generally contains low pH, total dissolved solids, total suspended solids,andtotalsolids.Theeffluentfromsugarmillscontains carbohydrates,nutrients,oil,grease,chlorides,sulfates,and heavymetals.Asaresult,allofthesecomponentscontribute significantlytotheirhighbiochemicaloxygendemand(BOD) andchemicaloxygendemand(COD).Sugarwastesaredull white in color and usually acidic in nature. The Total suspended solids affect the light intensity of water; suspendedsolidsarethecauseofsuspendedparticlesinside thewaterbodyinfluencingturbidityandtransparency.The pollutioneffectofsugarwasteisattributedtotheimmediate andhighoxygendemand.
The characteristics of a sugar effluent contain Temperature,Color,pH,DO,BOD,COD,totaldissolvedsolids, totalsuspendedsolids,totalsolids,chlorides,sulfate,oil& grease.Itdependslargelyonthequantityofsugarprocessed. Sugar mill effluent is highly polluting, which makes industriesdischarginguntreated/partiallytreatedeffluenta serious environmental threat. As part of the Indian government's environmental protection measures, very strict regulations are in place for effluent discharge. As a result, proper treatment methods are required to comply with effluent discharge standards. Most of the sugar industriesuseslimeasaneutralizerinETP.Thepurposeof thisexperimentistodeterminetheeffectivenessofSodium Hydroxideflakesasaneutralizerforeffluentshowninfigure 1anditseffectsonparameterssuchasTDS,TSS,TS,COD, andBOD.
2. METHODOLOGY
2.1 Sample Collection
Sampleswerecollectedfromtheinlettankinapolyethylene plastic can with a capacity of 10 liters. All samples were carriedtothelaboratoryandanalyzedwithin30minutes.All parameterswereanalyzedinaccordancewithMPCB.
2.2 Experimental Material
The effluent was obtained from The Saswad Mali Sugar FactoryLimited,Malinagar,Maharashtra,India.Thesodium hydroxide flakes used for effluent neutralization was analytical grade and was obtained from Thermo Fisher ScientificIndia.

2.2 Experiment Method
Beforetheneutralizationprocess,theeffluent’spHwas4.7, which was raised to 7.5 with 0.20 mg/L of NaOH as a neutralizer. pH was measured by a digital pH meter. TDS, TSS, TS, and COD were measured by Hach DR 3900 Colorimeter. BOD₅ at 20 °C was measured by dilution method.Theexperimentwasrepeatedthreetimesandthe averagevaluesweretakenasfinaldata.
3. RESULT AND DISCUSSION
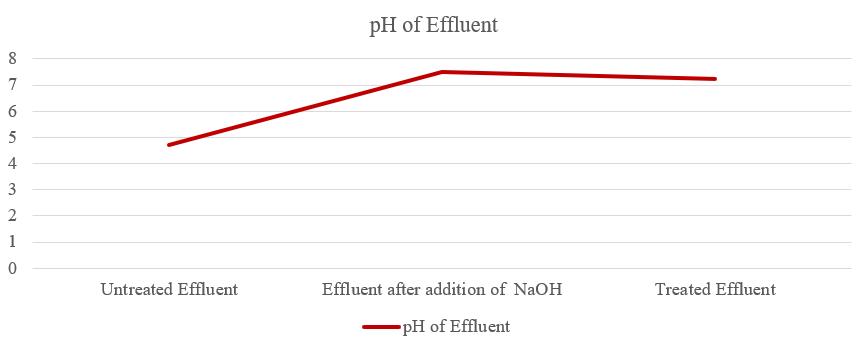
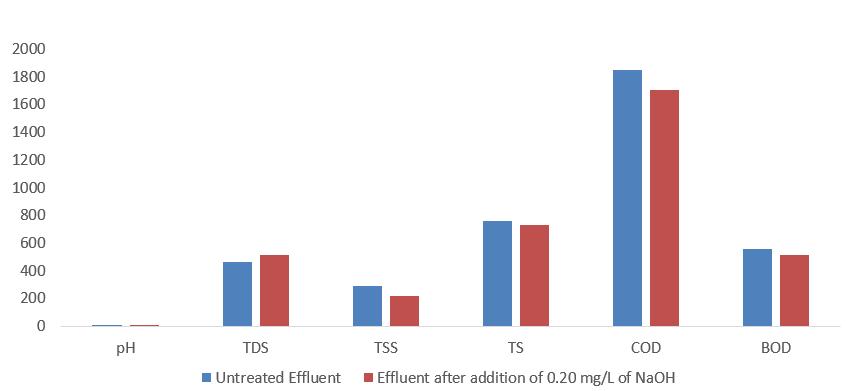
ThesugarmilleffluentcharacteristicaswellasMaharashtra PollutionControlBoardisshowninTable1.Basedonthe dataintable1,itisclearthatobservedparametersareoutof thelimitssetbyMPCB.Beingacidic,pHwhichislowthan 5.0,sugarmilleffluentalsocontainsahighlysuspendedsolid asindicatedbyhighturbidity,TDS,TSS,COD,andBOD.The COD, BOD, TSS, as well as turbidity of effluent, was decreased,whileTDSincreasedaftertheadditionofNaOHto neutralizeeffluentasshowninfigure2.TheincreaseinTDS wasmainlyduetotheincreaseinthelevelofdissolvedions present in the effluent which is derived from Na+ ions. Meanwhile,thedecreaseinTSSandturbiditywasduetoa smallnumberofsuspendedsolidsthatprecipitateduringthe additionoftheneutralizer.
3.1 pH
ThepHoftheuntreatedeffluentfromtheinlettankwas4.7, aftertheadditionofNaOHthepHwasraisedto7.5,andthe pHofthetreatedeffluentwas7.25asshowninfigure3.So, thepHoftreatedeffluentwaswithinthepermissiblelimit. UntreatedeffluenthasalowpHduetotheuseofphosphoric acid and sulfur dioxide in sugar cane juice clarification. A wide range in pH values can affect the rate of biological reaction and the survival of microorganisms. The pH of effluentisinfluencedbythepresenceorabsenceofdifferent ionic species. Consequently, soil quality can be strongly affectedbysucheffluent.
3.2 TDS
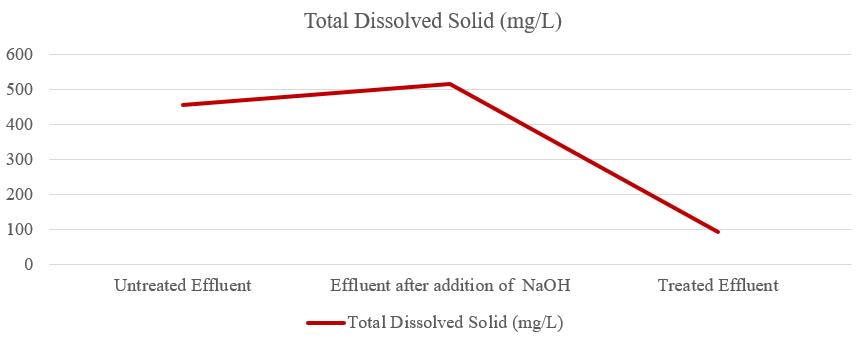
The total dissolved solids (TDS) of untreated effluent was 456mg/L,aftertheadditionofNaOHtheTDSofeffluentwas 516mg/L,andtheTDSoftreatedeffluentwas92.12mg/Las showninfigure4.So,theTDSoftreatedeffluentwaswithin thepermissiblelimit.Organicandinorganicmaterials,such asmetals,minerals,salts,andions,aredissolvedinwateras TDS.TheincreaseinTDSwasmainlyduetotheincreasein thelevel ofdissolvedionspresent in the effluent which is derivedfromNa+ions.
3.4 TS
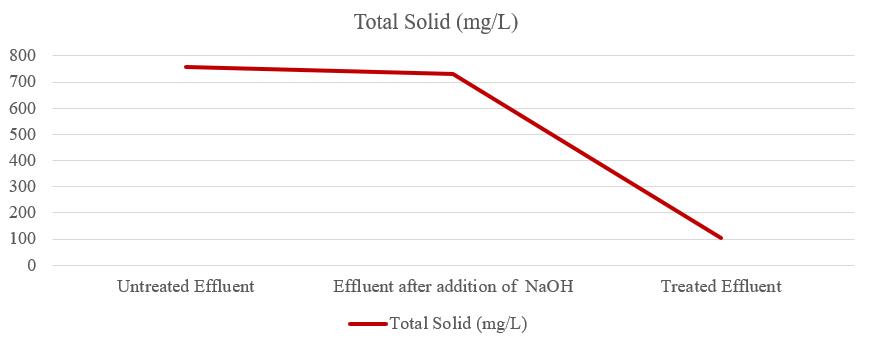
Thetotalsolidsinwaterarethesumofdissolvedsolidsand suspendedsolids.Thetotalsolids(TS)ofuntreatedeffluent was756mg/L,aftertheadditionofNaOHtheTSofeffluent was 732 mg/L, and the TS of treated effluent was 102.92 mg/Lasshowninfigure6.So,theTSoftreatedeffluentwas withinthepermissiblelimit.Solidsrefertoamatterthatcan be filtered or remains as residue after drying at a defined temperature on filter paper. n effluent, total solids are mainly made up of carbonates, bicarbonates, chlorides, sulfates,nitrates,calcium,magnesium,sodium,potassium, andmanganeseandorganicmattersilts.

3.3 TSS
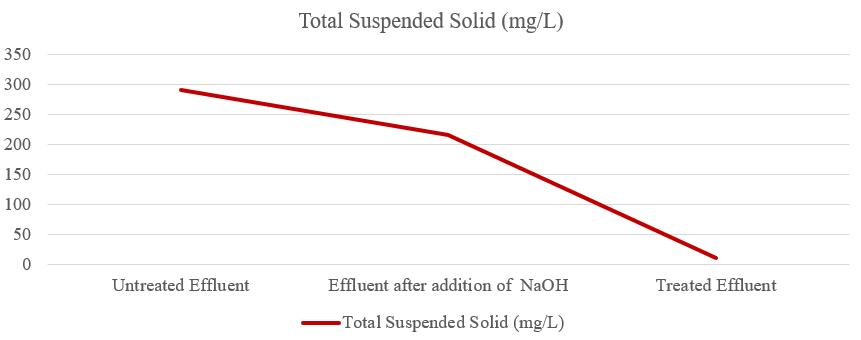
Thetotalsuspendedsolids(TSS)ofuntreatedeffluentwas 291mg/L,aftertheadditionofNaOHtheTSSofeffluentwas 216mg/L,andtheTSSoftreatedeffluentwas10.80mg/Las showninfigure5. So,theTSSoftreatedeffluentwaswithin thepermissiblelimit.TheTotalsuspendedsolidsaffectthe light intensity of water; suspended solids are the cause of suspended particles inside the water body influencing turbidityandtransparency.
3.5 COD
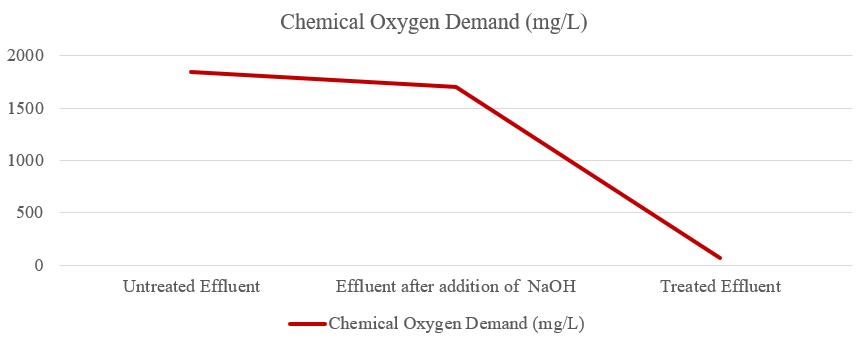
ChemicalOxygenDemand(COD)ofuntreatedeffluentwas 1848mg/L,aftertheadditionofNaOHtheCODofeffluent was1703mg/L,andtheCODoftreatedeffluentwas67.90 mg/L asshowninfigure7. So,theCODoftreatedeffluent was within the permissible limit. Sugar mill effluent has a high COD due to the presence of organic waste. COD tests measuretheoxygenrequiredtooxidizeorganicmatterusing strong oxidants. All organic compounds may be oxidized underacidicconditionsbystrongoxidizingagents.CODis useful for identifying toxic conditions and biologically resistantsubstances.
3.6 BOD
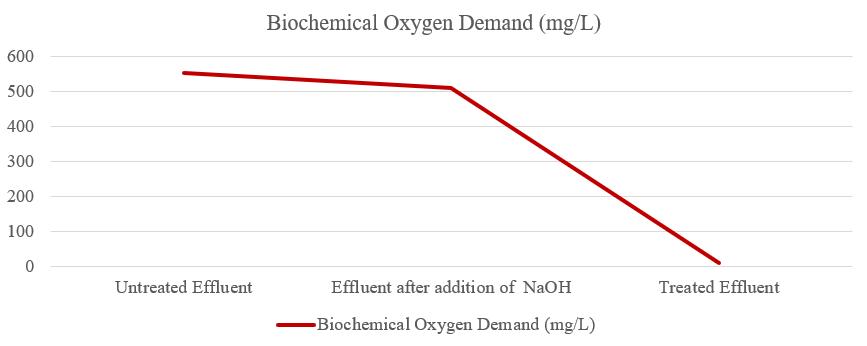
Biochemical Oxygen Demand (BOD) of untreated effluent was 554.4 mg/L, after the addition of NaOH the BOD of effluentwas510.9mg/L,andtheBODoftreatedeffluentwas 10.20 mg/L as shown in figure 8. So, the BOD of treated effluentwaswithinthepermissiblelimit.Itisdefinedasthe amount of oxygen required by microorganisms in wastewater to decompose organic matter under aerobic conditions.Asaresult,theBODismeasuredbyadecreasein dissolved oxygen values at a specific temperature over a specific time. Organic waste is oxidized by natural microorganismsatahighrate,resultinginhighBODlevels. Thegreaterthedecomposablematterpresent,thegreater the oxygen demand and the greater the BOD values. BOD measurestheamountoforganicmatterandoxygenusedto oxidizeinorganicmaterialsthatarereleasedintothewater.
TheamountrequiredforSodiumhydroxidetoraisethepH from4.7to7.5was0.20mg/L,twotothreetimeslowerthan conventional alkalis used for effluent neutralization. The removal efficiency of the Effluent Treatment Plant using Sodium hydroxide as a neutralizing base was 82%, 95%, 86%,96%,and98%forTDS,TSS,TS,COD,andBOD₅.From theaboveresults,itcanbeconcludedthattheparameters such as pH, TDS, TSS, TS, COD, and BOD obtained were within the permissible limits of Maharashtra Pollution ControlBroad(MPCB)andwerefittobedischargedinwater bodiesorcanbeusedforirrigation.
REFERENCES
[1] D.Shivappa,E.Puttaiah,andB.Kiran,“Physico-chemical characteristicsofSugarMillEffluentCurrentScenarioin Bhadravathi Taluk, Karanataka, India,” Journal of IndustrialPollutionControl,vol.23,no.2,pp.217–221, 2007.
[2] J.YadavandR.Pathak,“Analysisandphysico-chemical parameters of Sarvar Devla Sugar mill studiesn of effluents,”CurrentWorldEnvironment,vol.7,no.2,pp. 313–315,2012.
[3] L.MatkarandM.Gangotri,“Physicochemicalanalysisof sugar industrial effluents,” Journal of Industrial Pollution Contamination, vol. 18, no. 2, pp. 139–144, 2002.

[4] M. Salequzzaman, S. T. Islam, A. Tasnuva, M. Kashem, and M. M. Al Masud, “Environmental Impact of Sugar Industry - A Case Study on Kushtia Sugar Mills in Bangladesh,” Journal of Innovation Development Strategy,vol.2,no.3,pp.31–35,2008.
TheoverallefficiencyoftheeffluenttreatmentplantforTDS, TSS,TS,COD,andBODare82%,95%,86%,96%,and98%as showninfigure9.
[5] N.Billiappa,“Physico-chemicalpropertiesofsugarmill effluent,”JournalofBiologicalChemistry,vol.65,pp.79–82,1991.
[6] N. K. Chaurasia and R. K. Tiwari, “Physico chemical characteristicofSugarFactoryanddistilleryeffluent,” ScholarsResearchLibrary,vol.3,no.9,pp.4406–4408, 2012.
[7] P. Jadhav, G. Vaidyan, and S. Dethe, “Characterization and comparative study of cane sugar industrial waste water,”InternationalJournalofChemicalandPhysical Science,vol.2,no.2,pp.19–25,2013.
[8] P.SaranrajandD.Stella,“ImpactofSugarmilleffluentto environment and bioremediation: A Review,” World Applied Science Journal, vol. 30, no. 3, pp. 299–306, 2014.
[9] R. Senthil Kumar, R. N. Swamy, and K. Ramkrishan, “Pollution studies on sugar mill effluent– physicochemical characteristics and toxic metals,” Pollution Research,vol.20,no.1,pp.19–97,2001.
[10] W.A.SiddiquiandM.Waseem,“AComparativeStudyof SugarMillTreatedandUntreated118Physico-chemical AnalysisofTreatedandUntreatedEffluentsfromSugar Industry Effluent-A Case Study,” Orient Journal Chemistry,vol.28,no.4,pp.1899–1904,2012.

