A Review on Extracellular Matrix Synthesis Using Collagen Dressings with Plant Extract
Venkata Krishnan. S1 , Bidyut Bikash Pathak2 and R. Nirmala3*1PG Student, Department of Biotechnology, Hindustan College of Arts and Science, Affiliated to University of Madras, Padur, Chennai – 603 103, India
2Research Scholar, Department of Biotechnology, Hindustan College of Arts and Science, Affiliated to University of Madras, Padur, Chennai – 603 103, India

3Assistant Professor, Department of Biotechnology, Hindustan College of Arts and Science, Affiliated to University of Madras, Padur, Chennai – 603 103, India
***
Abstract - In order to improve wound healing procedures, the goal of this work was to create three-dimensional porous collagen composites (Col) containing polyphenol-rich wormwood extract and to evaluate how they interacted with human skin cells. Scanning electron microscopy was used to examine the ultrastructure of the scaffolds, and biodegradability and the release of bioactive chemicals were examined in a physiological setting. Using two in vitro experimental models, the interactions of composites in direct and indirect contact with human skin cells were assessed. In comparison to Col scaffold, ColWE scaffold had more porosity, a higher degree of swelling, and greater durability against enzymatic degradation. More wormwood extract content in composite scaffolds allowed for more effective regulation of polyphenolic release. Human dermal fibroblast and keratinocyte cell adhesion and proliferation were promoted by the ColWE 0.5 variant. The composite scaffold also promoted the production of skin extracellular matrix components. Our findings showed that ColWE composites with enhanced physical, chemical, and biological characteristics might be utilised in cutting-edge applications for wound healing.
Key words: Extracellularmatrix,Scaffold,Collagendressing,Plantextract
1. INTRODUCTION
Skin wounds have a lengthy healing process that involves four overlapping stages: inflammation, granulation tissue development,reepithelialization,andextracellularmatrixremodelling(ECM).Thenaturalhealingprocessmightbehampered byexternalcircumstanceslikebacterialinfectionsorinternaloneslikethepatient'soldageorillness,whichcanresultin chronicwounds[1].Tospeedupthehealingprocesses,avarietyofpassive(gauzes,films,hydrocolloids,hydrogels,andfoams) and active (biocomponents loaded in polymeric matrix) dressings were evaluated on various skin wound types [2]. The primaryfunctionsofawounddressingaretopreservemoisturewhileremovingexcessivequantitiesofexudatefromtheinjury site,topermitgaseousexchange,andtoofferdefenceagainstbacterialinfection.Moreover,awounddressingmustbesimpleto remove from the wound, biodegradable, and biocompatible [3,4]. The primary structural element of ECM and the most researchednaturalpolymerfortissueengineeringapplicationsiscollagen(Col)[5-7].Becausetotheirhighbiocompatibility, simpleadhesion,capacityforswelling,andprotectionofthewoundbed,collagenicdressingshavebeenusedtospeedupthe healing of skin wounds [8].Col matrices further offered an ideal three-dimensional (3D) microenvironment for cellular adhesionandproliferationatthesitesoflesions,encouragingthecreationofgranulationtissue,re-epithelialization,andnew ECMsynthesisaspartofthewoundhealingprocess.Plantextractsandtheirphysiologicallyactiveconstituentspromotedthe healingofskinwounds[9].Wormwood,orArtemisiaabsinthiumL.,haslongbeenusedasafebrifuge,antiseptic,antifungal, and antibacterial agent [10,11]. Wormwood polyphenolic extracts also shown antioxidant and free radical scavenging properties,whichmayimprovethehealingprocessofwounds[12-14].
Duetotheirpharmacologicalactioninducedbyregulatedreleaseofactivemoleculesandlongercontactextentwith skinlesions,plantextract-polymericconstructionsarecurrentlyreceivingalotofinterestinwoundhealingapplications[1517].Recently,sheetsmadeofcollagensthatwereloadedwithplant-derivedrestorativechemicalsorwholeplantextractswere created for use in skin tissue engineering. As comparison to Col scaffolds [16-18], Col dressings with polyphenols from Hamamelisvirginianahadabetterabilitytoinhibitchronicwoundenzymesincludingmyeloperoxidaseandcollagenase.Rats withinfectedcutaneouswoundsweretreatedwith3DspongesofColfilledwithtriphalaherbalextract,leadingtorapidwound closureandtissueregeneration[19,20].Col-chitosanscaffoldsaddedwithAloeVerageldisplayedbetterphysicochemicaland biologicalcharacteristics,whereasCol-matriceswithAstragaluspolysaccharideswereproducedaspotentialwounddressings withangiogeniccapabilities.Thecapacityforrecruiting,attachment,andproliferationoffibroblasts[21,22].
1.1 Ultrastructure of ColWE scaffolds
ColWEscaffoldshada3Dsponge-likeappearance,whichistypicaloffreeze-driedpolymericmatrix.Theirsurfacewas shownbySEM examinationstohavea randommicroporousstructurewithtinyholes,comparabletoColscaffold.ColWE scaffolds'transversecrosssectionrevealedaconsistentporositystructure,comparabletotheColscaffold.TheColWEscaffolds' wholestructurewasmadeupofinterconnectedpores,whosediametersrangedfrom50to600m.Theultrastructure,pore size, and morphology of the composite scaffolds were comparable to those of the Col scaffold, suggesting that before lyophilization,ColgelandWEformedahomogenouscombination.
Watercrystalssublimatedduringthefreeze-dryingprocess,whichledtotheformationandshapeofpores.Inthis work,lyophilizationwasemployedtoachieveoptimalcelltypecontactandregulatedreleaseofbioactivecompounds.Ithas beendemonstratedthat,duetotheir3Dporousnature,freeze-driedbiomaterialsgrownwithcellsassurenutrientsdiffusion, promotecellproliferation,andproducemoreECMthan2Dmaterialsorloosehydrogels[23].Furthermore,byalteringthe freezingtemperaturebetween80°Cand20°C,theporesizeandmicrostructureofthesematerialsmaybefurthercustomised [24].Ascaffold'sabilitytoaccommodateparticularporediametersfavourscellinfiltration,adhesion,andproliferation,ithas beenshown[25].
Skinhasthesamenon-homogeneityinporesizeasthecompositematerials[26].Microporesimprovethemechanicsof thescaffoldandprovidetargetedcellmigrationduringtissueregenerationanddefectcorrection,whereasmacroporesinthis complextissueoftenserveasspacesfortissuedevelopmentandvascularization[27].Contrarily,thelyophilizationprocedure andvacuumexposureoftheupperhalf,whilethelowerpartwasindirectcontactwiththeplasticmould,wereresponsiblefor thediscrepanciesinsurfaceandinsideporemorphology[28].
1.2 Physico-chemical characterization of ColWE scaffolds

WaterwasusedasthesolventinaliquiddisplacementapproachtoassessthetotalporosityoftheColWEscaffold variations.Littlewatermoleculesdispersedintothescaffolds'variousporediametersbeforebeingcompelledbypressureto quicklyfilltheemptyspace.ThemostlyhydrophobicColmoleculeandthepoorwatersolubility,polyphenol-richWEinhibited theswellingofthesamplesduringtheincubationperiod.TheoutcomesaredisplayedinTable1.ColWE0.25andColWE0.5% hadaporosityof80%,whereasColWE1andColWE2.5hadaporosityof70%.Colscaffoldthathadbeenfreeze-driedhada 97% porosity value (Table 1).As comparison to Col scaffold, incorporation of increasing WE caused a proportionate and substantial (p<0.05) reduction in porosity. Nevertheless, ColWE scaffolds with porosity exceeding 70% may be more advantageousforcellcolonisationanduseinskintissueengineering[23].Thecross-linkingbetweenthechainsofColandthe polyphenolsandtheproductionofmanyhydrogenbondsthatchangetheultra-structureoftheporousscaffoldmaybeto blameforthereductioninporosity[27].
Col scaffoldshavethecapacityto expand,increasing their volume byat least1500%abovethat of their starting volume[29].CompositematerialsmadeofColloadedwithincreasingamountsofpolyphenol-richWEthathadalotoffree hydroxylgroupsonthehydrophobicbackboneshouldhavebetterswellingdegreeandincreasedhydrophilicity[31].
Inthisregard,theColWEcompositevariations'swellinglevelswereexamined,andthefindingsareshowninTable1. ExceptwithColWE2.5,thevaluesforColWEdressingswereconsiderably(p0.05)higherthanthoseforColscaffold(Table1) WhentheWEconcentrationincreased,theswellingdegreedecreasedfrom2342%forColWE0.25%to1936%forColWE2.5. Thismaybeasaresultoftheformationofcross-linksbetweenthechainsofColandpolyphenols,whichwouldreducethe amountoffreeaminoandhydroxylsidegroupswithhydrophilicqualitiesandlowerswellingdegree.Thisvariancereflecteda reductioninporosity.ColWE0.25,ColWE0.5,andColWE1versions,however,shouldbeabletohelpexudateabsorptionatthe woundsitesbecausetotheirbetterswellingcapabilities.Similarstudieshavefoundthataddinghigherconcentrationsof vegetalpolysaccharidestocreateacollagenichybridmaterialincreasesthewaterabsorptionofCol-polyphenolicbiomaterials comparedtoCol20anddecreasesswellingdegree[31].
Collagenicwounddressingsaresusceptibletoenzymaticbreakdowninvivo.Thebiodegradabilitytestinthisstudy wasconductedusingcollagenasetypeIA,whichisonlycapableofcleavingthe-X-Gly-Prosequencefromconnectivetissue components.Accordingtothefindings,ColWEscaffoldsdeterioratedtoalesseramountthanColmatrix(35%
86%).(Table1). Also,increasedWEconcentrationsledtoimprovedscaffoldresilienceagainstcollagenaseassault.ColWE0.25wastherefore 86%degraded,comparedtoColWE1scaffold's50%degradationandColWE2.5's35%degradation(Table1).Thismaybe explainedbythepolyphenolicextract'shighconcentrationofhydroxylandcarboxylgroups,whichfavoursmanyhydrogen bondswithproteins,particularlyCol[33].SimilaroutcomeswerepreviouslyattainedwithColspongesloadedwithHamamelis virginianapolyphenols,whichdemonstratedenhancedstabilitytowardschronicwoundenzymes[19].
1.3 In vitro release of biologically active compounds

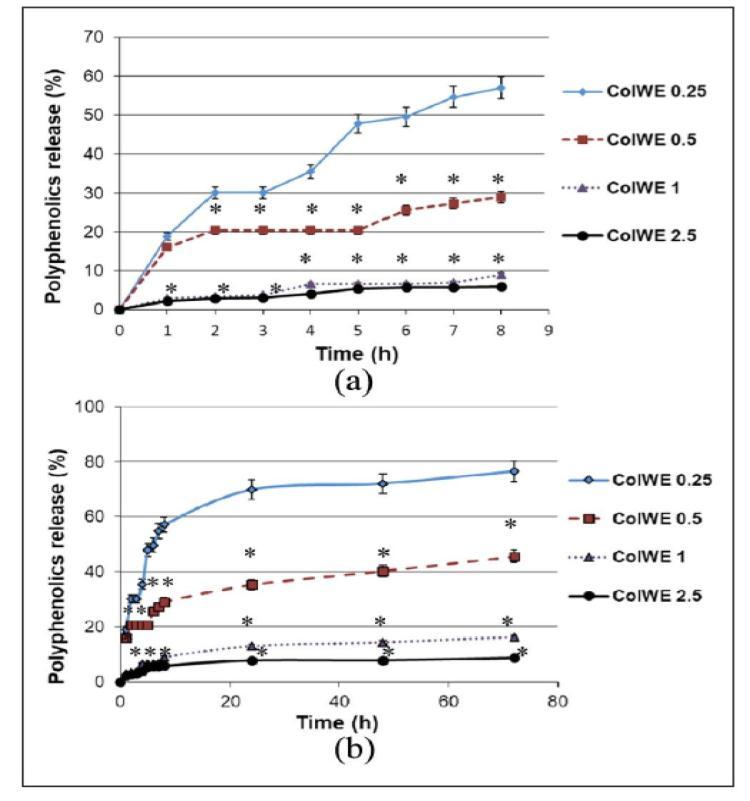
PBSpH7.4at37°CwasusedtostudythepolyphenolicsreleasefromColWEporousscaffoldsincircumstancesthat mimickedaninvivoenvironment.Theoutcomesaredisplayed.Theresultsshowedthatwithinthefirst8hoursofincubation, significantlevelsofpolyphenolicsweregraduallyreleased.Between8and24hofincubation,theslopeofthereleaseprofile reduced,andadditionalincubationledtoaplateauinthereleaseofpolyphenolics.Attheconclusionoftheincubationperiod, the percentage of released polyphenolic compounds for ColWE 0.5 and ColWE 0.25, respectively, achieved high levels of 45.42%and 76.43%. Lower percentagesofphenolics(16.13%and 8.85%, respectively) werereleasedin the physiologic environmentbyColWE1andColWE2.5scaf-folds.Thebiodegradabilityofthescaffoldsmaybetoblamefortheresults,since slowerdegradingvariationsreleasedalesserpercentageofbioactivechemicals.Theactuallevelsofphenolicsproducedinthe physiologicenvironment,however,werecontainedwithinasmallrange.ThecomponentsthatwerenotinvolvedinphysicochemicalinteractionswithColmoleculespresumablyaccountedforthiscommonalityinreleasedquantities[29].
1.4 ColWE scaffold interaction with human skin cells i. ColWE effect on cell proliferation and viability
InaccordancewithSRENISO10993-5/2009,apreliminarycytotoxicitytestofColWEvariationswascarriedoutina cultureoffibroblasts(L929clonecellline).After24hoursofculture,thefindingsshowedthatColWE0.25andColWE0.5had goodbiocompatibility(>80%cellviability),whereasColWE1hadmoderatecytotoxicity(>70%cellviability),andColWE2.5 varianthadlowcytotoxicity(30%cellviability).Hence,onlyColWE0.25,ColWE0.5,andColWE1werechosenforadditional cellcultureresearch.ThehighphenolicsamountreleasedintothecellculturemediumandinadequateColquantitytoprotect skincellsmaybethecauseofColWE2.5'scytotoxicity.Moreover,anabruptriseinthereleaseprofilemaybe relatedtoan increaseinthesurface-to-volumeratioofthesampleutilisedincellcultureexperiments[33].
Inordertoassesscellproliferation,fibroblastsandkeratinocytes,twokindsofhumanskincells,werecultivatedinclose proximitytoColWEscaffolds.Thefindingsindicatedthatduringthecourseofthewholeculturetime,thenumberoffibroblast cellssteadilygrew.Thecellquantitywasconsiderably(p0.05)largerinColWE0.25andColWE0.5variants,duringeachtime ofculture,andthecellsproliferatedtoagreaterdegreein3DColWEscaffoldsthanonplastic(control).At7daysofincubation, ColWE1scaffoldpermittedcellproliferationtoasimilarlevelasColscaffold,butgreaterthanthe2Dcontrolgroup.
NochangeswerefoundbetweenthetestedgroupsaftertwodaysofcultureforHaCaTkeratinocytecells.Theresults revealedthatColWE0.5(46.9104cells)hadseenanextensiveproliferationofkeratinocytesafter5daysofincubation.ColWE 0.25andColWE1encouragedcelldevelopmenttoagreaterextentthanColscaffold(28.5104cells)andcellsgrownonplastic (18.2104cells,respectively)(37.2104and34.6104cells,respectively)(control).Following7daysofculture,therewerealso noticeablymorekeratinocytecellsthanintheCol scaffold(p0.05).Nevertheless,it fell inall examinedgroups,including control,mostlikelyasaresultofcelloverpopulation,whichquicklyateupthenutrientsavailable.
Theirstudiesshowedthat,incomparisontoColscaffold,someColWEcompositeversions,suchasColWE0.25andColWE 0.5,weremoreeffectiveas3Dscaffoldsforskincells,particularlyforkeratinocytes.OnlyintheHaCaTkeratinocytecellculture didColWE1dressingoutperformCol scaffold.ColWEcompositesmayhavea stimulatingimpactbecauseoftheirporous design,whichmadeitpossiblefornutrientsfromtheculturemediumtobeefficientlyabsorbed.Moreover,thegrowthofboth
typesofhumancellswasaidedbysmallquantitiesofWEincludedincompositesandphenolicsdischargedintothebiological medium.EarlierresearchdemonstratedthatthepolyphenolicsfoundintheArtemisiagenusmightpromotethegrowthofskin cells[34].Ontheotherhand,Col,whichhasadistincttriplehelicalstructure,isknowntoencouragecutaneousfibroblastcell adhesionandproliferationwhenpreparedas3Dscaffolds[35].TherewerenoreportsofWE-loadedColclothsinteractingwith skincells.
ThesignificantnumberoflivecellsthatcolonisedColWEscaffoldswithoutcytotoxicitywasconfirmedbyfluorescence microscopymeasurements.Keratinocytesformedisolatedcoloniesonthesurfaceofscaffolds,whereasdermalfibroblasts movedthroughoutthewholeconstructionandinfiltratedit.
ii. ColWE effect on ECM synthesis by skin cells

Oncedermalfibroblastsweregrowninthepresenceofcompositescaffolds,thetotalamountofcollagenssecretedinthe culturemediumwasfirstcalculated.Incomparisontocontrolcellculture,theresultsshowedthatColWE0.25andColWE0.5 increasedcollagensynthesisbythriceandfourfold,respectively.Moreover,theresultsdifferedconsiderably(p0.05)with thoserecordedforColscaffold.Comparedtocontrolcells,fibroblastcellsproducedmorecollagenwhenexposedtoColWE1 andColscaffolds,whiletheeffectsweremodest.SimilaroutcomeswereobtainedwhenHaCaTkeratinocyteswereculturedon ColWEscaffoldstoproducecollagen.
All of these findings showed that, due to specific WE concentrations; ColWE scaffolds stimulated the manufacture of collageninhumanskincells.EarlierresearchshowntheabilityofflavonoidglycosidesfromtheArtemisiagenustoincreasethe synthesis of Col in human skin fibroblast cultures, including isoquercitrin, quercetin-3-O-d-glucoside, quercetin-3-Orhamnoglucoside, and isorhamnetin-3-glucoside [36-38]. Dermal fibroblasts cultured in the presence of ColWE scaffolds producedfibronectin,anotherimportantcomponentoftheECM.ColWE0.5andColWE0.25versionsconsiderably(p0.05) boosteditsproductioncomparedtoColscaffoldandthecontrolgroup.
ColWE1scaffold-cultivatedfibroblastsproducedalmostthesameamountoffibronectinastheColscaffoldgroupdid. ColWE0.5scaffoldconsiderably(p0.05)increasedtheamountoffibronectinthatwasgeneratedinHaCaTkeratinocytecells comparedtotheColscaffoldgroupandcontrolcells.ColWE0.5scaffoldwasshownbycellculturefindingstobeidealforskin cells'proliferationandmetabolism,increasingthesynthesisofECM[39].
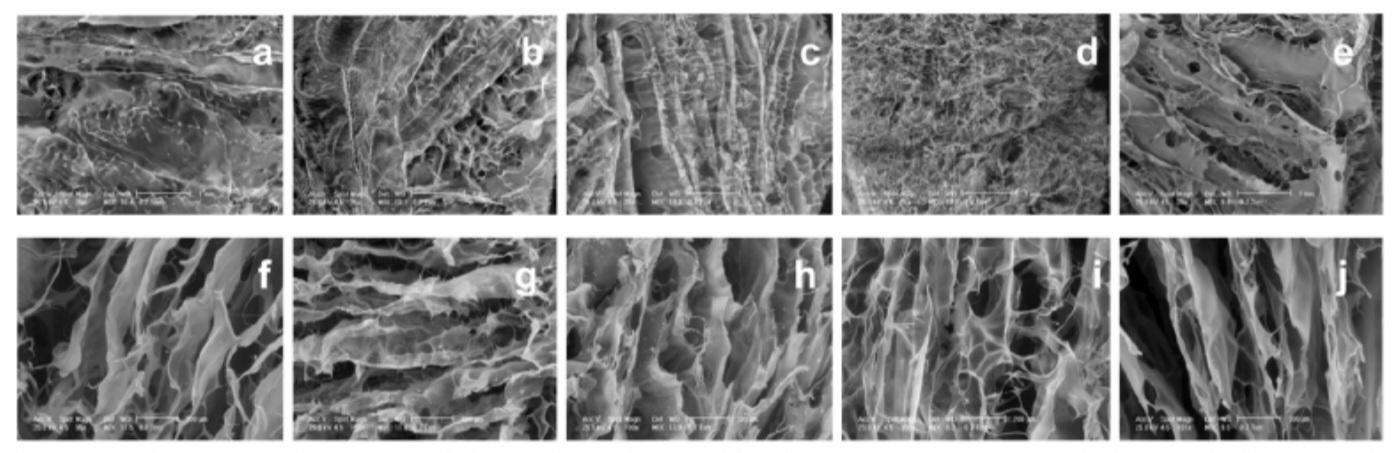
Figure 2. Polyphenolicsreleaseprofileforcompositescaffolds,after(a)8hand(b)72hofincubationinbiomimetic conditions(salinebuffer,pH7.4,37°C).Totalquantityofplantextractineachsamplewasconsidered100%.Theresults wereexpressedasmean±SD(n=3).*p<0.05,comparedtocompositescaffoldColWE0.25[39].
REFERENCES
1.BarrientosS,StojadinovicO,GolinkoMS,etal.Growthfactorsandcytokinesinwoundhealing.WoundRepairRegen2008; 16(5):585–601.
2.MogosanuGDandGrumezescuAM.Natural andsyntheticpolymersforwounds andburnsdressing.IntJ Pharm2014; 463(2):127–136.

3.BoatengJS,MatthewsKH,StevensHN,etal.Woundhealingdressingsanddrugdeliverysystems:areview.JPharmSci2008; 97(8):2892–2923.
4. Moura LI, Dias AM, Carvalho E, et al. Recent advances on the development of wound dress-ings for diabetic foot ulcer treatment areview.ActaBiomater2013;9(7):7093–7114.
5.MalafayaPB,SilvaGAandReisRL.Natural-originpolymersascarriersandscaffoldsforbiomoleculesandcelldeliveryin tissueengi-neeringapplications.AdvDrugDelivRev2007;59(4–5):207–233.
6.DongCandLvY.Applicationofcollagenscaf-foldintissueengineering:recentadvancesandnewperspectives.Polymers 2016;8(2):42–62.
7. Oh HH, Uemura T, Yamaguchi I, et al. Effect of enzymatically cross-linked tilapia scale col-lagen for osteoblastic differentiationofhumanmesenchymalstemcells.JBioactCompatPol2016;31(1):31–41.
8.ChattopadhyaySandRainesRT.Biomaterialsforwoundhealing.Biopolymers2014;101(8):821–833.
9.PirainoF andSelimovicS. Acurrentview offunctional biomaterialsfor woundcare, molecu-larandcellulartherapies. BiomedResInt2015;2015:403801.
10.BudovskyA,YarmolinskyLandBen-ShabatS.Effectofmedicinalplantsonwoundhealing.WoundRepairRegen2015; 23(2):171–183.
11.BoraKSandSharmaA.ThegenusArtemisia:acomprehensivereview.PharmBiol2011;49(3):101–109.
12.Canadanovic-BrunetJM,DjilasSM,CetkovicGS,etal.Free-radicalscavengingactivityofwormwood(Artemisiaabsinthium L)extracts.JSciFoodAgric2005;85:265–272.

13.BoraKSandSharmaA.Evaluationofantioxi-dantandfree-radicalscavengingpotentialofArtemisiaabsinthium.PharmBiol 2011;49(1):1216
1223.
14.ErelSB,ReznicekG,SenolSG,etal.AntimicrobialandantioxidantpropertiesofArtemisiaL.speciesfromwesternAnatolia. TurkJBiol2012;36:75–84.
15. Naseri-Nosar M, Farzamfar S, Salehi M, et al.Erythropoietin/Aloe Vera-releasing wet-elec-trospun polyvinyl alcohol/chitosansponge-likewounddressing:invitroandinvivostudies.JBioactCompatPol2017;33:269–281.
16. Andreu V, Mendoza G, Arruebo M, et al.Smart dressings based on nanostructured fibers containing natural origin antimicrobial,anti-inflammatory,andregenerativecompounds.Materials2015;8(8):5154–5193.
17. Das U, Behera SS, Singh S, et al. Progress in the development and applicability of potential medicinal plant extractconjugatedpolymericconstructsforwoundhealingandtissueregen-eration.PhytotherRes2016;30(12):1895–1904.
18.GasparA,CraciunescuO,MoldovanL,etal.Newcompositescollagen-polyphenolsaspoten-tialdressingforwoundcare. RomJBiochem2012;49(2):173–181.
19.FranceskoA,RocasalbasG,TourinoS,etal.Cross-linkedcollagenspongesloadedwithplantpolyphenolswithinhibitory activitytowardschronicwoundenzymes.BiotechnolJ2011;6(10):1208–1218.
20.KumarMS,KirubanandanS,Sripriya R,etal.Triphala incorporatedcollagensponge a smartbiomaterial forinfected dermalwoundhealing.JSurgRes2010;158(1):162–170
21.YaoC,LiA,GaoW,etal.Improvingtheangio-genicpotentialofcollagenmatricesbycovalentincorporationofAstragalus polysaccharides.IntJBurnsTrauma2011;1(1):17–26.
22. Jithendra P, Rajam AM, Kalaivani T, et al. Preparation and characterization of Aloe Vera blended collagen-chitosan compositescaffoldfortissueengineeringapplications.ACSApplMaterInterfaces2013;5(15):7291–7298.
23.Rodriguez-VazquezM,Vega-RuizB,Ramos-ZunigaR,etal.Chitosananditspotentialuseasascaffoldfortissueengineering inregenerativemedicine.BiomedResInt2015;2015:821279.
24.MurphyC,HaughMandO’BrienF.Theeffectofmeanporesizeoncellattachment,prolif-erationandmigrationincollagenglycosami-noglycanscaffoldsforbonetissueengineering.Biomaterials2010;31(3):461–466.
25.XuHHandSimonCG.Self-hardeningcalciumphosphatecement-meshcomposite:reinforce-ment,macroporesandcell response.JBiomedMatRes2004;69(2):267–278.
26.LohQLandChoongC.Three-dimensionalscaffoldsfortissueengineeringapplications:roleofporosityandporesize.Tissue EngPartBRev2013;19(6):485
502.
27. Macchetta A, Turner IG and Bowen CR. Fabrication of HA/TCP scaffolds with a graded and porous structure using a camphene-basedfreeze-castingmethod.ActaBiomater2009;5(4):1319–1327.
28.Jarquin-YanezK,Arenas-AlatorreJ,Pinon-ZarateG,etal.StructuraleffectofdifferentEDCcrosslinkerconcentrationin gelatin-hyaluronicacidscaffolds.JBioengBiomedSci2016;6:182.
29.KrishnamoorthyG,MadhanB,SadullaS,etal.StabilizationofcollagenbytheplantpolyphenolicsAcaciamollissimaand Terminaliachebula.JApplPolymSci2008;108:199–205.
30.AlbuMG,TitorencuIandGhicaMV.Collagen-baseddrugdeliverysystemsfortissueengineering.In:PignatelloR(ed.) Biomaterialsapplicationsfornanomedicine.1sted.Rijeka:InTech,2011,pp.333–358.
31.CheirmaduraiK,ThanikaivelanPandMuraliR.Highlybiocompatiblecollagen-Delonixregiaseedpolysaccharidehybrid scaffoldsforantimicrobialwounddressing.CarbohydrPolym2016;137:584
593.
32. Madhan B, Subramanian V, Rao JR, et al. Stabilization of collagen using plant polyphe-nol: role of catechin. Int J Biol Macromol2005;37(1–2):47–53.
33.RajuPN,PrakashK,RaoTR,etal.Effectoftab-letsurfaceareaandsurfacearea/volumeondrugreleasefromlamivudine extendedreleasematrixtablets.IntJPharmSciNanotechnol2010;3(1):872–876.

34. Zhang Y, Wang J, Cheng X, et al. Apigenin induces dermal collagen synthesis via smad2/3 signaling pathway. Eur J Histochem2015;59(2):98–106.
35.MoldovanL,BuzgariuW,CraciunescuO,etal.Preparationofporouscollagenmatricesandtheirinteractionwithdifferent celltypes.In:GruevB,NikolovaMandDonevA(eds)ProceedingsoftheBalkanscientificconferenceofbiology.(1sted). Plovdiv:PlovdivUniversityPress,2005,pp.671
681.
36.ZhengGQ.Cytotoxicterpenoidsandfla-vonoidsfromArtemisiaannua.PlantaMed1994;60(1):54–57.
37.IvanescuB,VlaseL,CorciovaA,etal.HPLC-DAD-MSstudyofpolyphenolsfromArtemisiaabsinthium,A.annua,andA. vulgaris.ChemNatComp2010;46(3):468–470.
38.NazarukJandGalickaA.TheinfluenceofselectedflavonoidsfromtheleavesofCirsiumpalustre(L.)Scop.oncollagen expressioninhumanskinfibroblasts.PhytotherRes2014;28(9):1399–1405.
39.EnhancedextracellularmatrixsynthesisusingcollagendressingsloadedwithArtemisiaabsinthiumplantextractAlexandra Gaspar-Pintiliescu,Ana-MariaSeciu,FlorinMiculescu,LuciaMoldovan,ElenaGaneaandOanaCraciunescu;JournalofBioactive andCompatiblePolymers 1–13.
