Evaluating the Consequences of Chemical Accidents in Fertilizer Plants using ALOHA Program
Tejas RawoolDepartment of Fire Technology & Safety Engineering, IPS Academy Institute of Engineering & Science, Indore, India ***

Abstract - The fertilizer industry is an essential component of modern agriculture, providing critical nutrientsforcrops to growandproduceyields. However, the production, storage, and transportation of fertilizers can also pose significant risks to workers and communities due to the potential for accidents and incidents. Fertilizer industry accidents can result in severe injuries, loss of life, environmental damage, and economic impacts. In this paper, simulations of chemical accidents are presented. The accident was caused by an uncontrolled release of ammonia from a storage tank in the fertilizer plant. The simulation was conducted using the ALOHA software, which anticipates the development of the accident and determines the areas of risk and safety. The experiment is based on the physical and chemical properties of the substance involved in the accident, and the paper discusses the various scenarios that could occur under the most unfavorable atmosphericconditions.
Key Words: Fertilizer, ALOHA, Simulation, Risk, Uncontrolled.
1. Introduction
Safety should be a top priority in the fertilizer industry, especiallyinhigh-riskareaslikeammonia-ureaplantsthat operate under intense pressure and temperature. So, what's the first step in ensuring industrial safety? It's identifyingpotentialhazards,whichmeanslookingforany undesired events that could lead to a hazardous situation and the mechanisms that could cause them. Once we've identified these hazards, we need to assess the potential damage they could cause to the surrounding area and comeupwithwaystolowertheriskasmuchaspractically possible. In order to effectively address potential safety hazards associated with the storage and handling of ammonia,theadvancedsoftwareALOHAwillbeemployed to conduct sophisticated simulations. Upon careful evaluation and analysis of the simulation results, appropriate preventive measures can be taken by the relevant authorities to ensure the safety of personnel and theenvironment.
2. Theoretical part
According to the World Health Organization, a chemical accident inafertilizerindustryisanalarmingoccurrence
that poses a significant threat to public health and the environment. It refers to the uncontrolled release of a toxic substance, which has the potential to cause harm, either immediately or over an extended period. Chemical incidentscanoccurduetonaturalcauses,suchasvolcanic eruptions, earthquakes, or hurricanes. Conversely, they canalsoariseasaresultofhumanerror,systemfailure,or deliberate acts of sabotage, terrorism, or warfare. The severityofachemicalincidentcanvary,dependingonthe nature, quantity, and concentration of the substance released, the environmental conditions at the time of release, and the proximity of humans and animals to the source of contamination. The incident can be sudden and acute, leading to rapid onset of symptoms and potentially fatal consequences. Alternatively, it can have a delayed onset, with the harmful effects not becoming apparent until days, weeks, or even years after exposure. This type of incident is commonly known as a "silent" release of a chemical and can pose long-term health risks to those exposed. The range of chemical incidents can also vary from small-scale releases, which can typically be handled by local emergency services, to full-scale major emergencies, which require a coordinated response from multipleagenciesatlocal,national,andeveninternational levels. The latter can involve large-scale evacuations, decontamination efforts, and medical treatment for affected individuals, all of which require careful planning, resourcemobilization,andcommunication.Theincreasing production and use of chemicals worldwide have raised concerns about the potential health and environmental impacts of these substances. Consequently, the health sector has had to expand its traditional roles and responsibilities to address the public health and medical issues associated with chemical usage and its health effects.Thisincludesprovidingexpertiseandguidance on chemical safety, monitoring and assessing the health impacts of chemical exposures, developing emergency response plans, and supporting research on the health effectsofchemicals.Inthisregard,thehealthsectorplays a critical role in ensuring the protection of public health andtheenvironmentinthefaceofchemicalincidents.
2.1 Ammonia

Table -1: Physical-chemicalcharacteristicsofammonia
Chemicalformula NH3
Name Ammonia,Azane(IUPAC)
Molarmass 17.031g/mol
Apperance Colorlessgas
Odor Pungentodor
Density 0.76g/LatSTP
Meltingpoint -77.37 0C
Vapourpressure 1003kPa(at250C)
Auto-ignition Temperature 6510C ExplosiveLimits 14.8-33.5%
Ammoniaisaninorganiccompound,comprisedofasingle nitrogenatomthatiscovalentlybondedtothreehydrogen atoms. It is a potent inhibitor of amidase and neurotoxin, and is both synthetically manufactured and naturally produced through bacterial processes and the decomposition of organic matter. Its uses span across a wide range of industries, including its applications as a refrigerant and fertilizer. Ammonia is identified by its characteristic pungent odor and is commonly found in a colorless gas or compressed liquid form, with exposure typically occurring through inhalation, ingestion, or contact. Both long-term inhalation of low concentrations and short-term inhalation of high concentrations of ammonia vapors can result in adverse health effects. Despitethis,ammonia continuesto beusedasa fertilizer, refrigerant,andinthemanufactureofotherchemicals.Its rateofonsetisimmediateanditpersistsforminutes,with an odor threshold of 17 parts per million. It is worth noting that ammonia is also utilized in explosives manufacture, pesticides, and the detergents industry, and posesa rangeofotherhazardsaswell.Thelabel depicted in Figure 1 serves as a comprehensive reference to identify and highlight all of the common hazards associatedwiththestorageandhandlingofammonia.
In its anhydrous form, ammonia is a clear and colorless gas that can be shipped as a liquid under its own vapor pressure. Its liquid form is known to be highly dense, weighinginatapproximately6poundspergallon.Contact with the unconfined liquid can cause severe frostbite. Whilethegasistypicallyregardedaslightlyflammable,it has been known to ignite within certain vapor concentrationlimitsandwithstrongignitionsources.The presence of oil or other combustible materials can significantlyincreasetheriskoffire.Itisimportanttonote that,despite beinglighterthanair,thevaporsfroma leak
initiallyclingto theground.Prolonged exposuretofire or heatcancauseviolentrupturingandrocketing.
One of the key features of ammonia is its combustion characteristics.Ammonia doesnotburnreadilyorsustain combustion under most conditions. This is due to several factors, including its low heat of combustion, high autoignition temperature, and narrow flammability range. In other words, ammonia requires a specific mixture of fuel andairto burn,whichlimitsitspotential asa fuel source. However,whenammoniadoesburn,itproducesadistinct flamethat is paleyellowish-green incolor. Thisflameis a result of the chemical reaction between ammonia and oxygen, which forms nitrogen and water. This reaction is exothermic,meaningitreleasesenergyintheformofheat. Thestandardenthalpychangeofcombustionforammonia is -382.81 kJ/mol, which indicates the amount of energy released per mole of ammonia combusted. Interestingly, the combustion of ammonia can also produce nitrogen oxides, which are important industrial chemicals. When ammonia reactswithoxygeninthepresence ofa catalyst, itcanproducenitricoxide(NO)andwater.Thisreactionis important in the production of nitric acid, which is a key ingredientinfertilizers,explosives,andotherproducts.
4NH3+3O2→2N2+6H2O(gΔH°r=−1267.20kJ),
Nitrogen oxides can also react with other compounds in the atmosphere to form smog and acid rain, which can have negative effects on human health and the environment.

2.2 Causes of Ammonia Leakage
To Identify the causes of leakage of ammonia, the root cause analysis method was used. A root cause analysis allows an employer to discover the underlying or systemic,ratherthanthegeneralizedorimmediate,causes of an incident. Correcting only an immediate cause may eliminate a symptom of a problem, but not the problem itself.Itisimportanttoconsiderallpossible“what,”“why,” and “how” questions to discover the root cause(s) of an incident. This method was used to identify seven basic causesofammonialeakage,whicharelistedbelow.
1) Corrosion: Corrosion can occur through a variety of mechanisms, but one common cause is the combination of oxygen, ammonia, stress, and carbon steel. When carbon steel is exposed to an environment containing oxygen and ammonia, a reaction can occur that produces ammonium compounds. These compounds can react with the metaltoformironoxide,whichisatypeofrust.This reaction is accelerated by the presence of stress on the metal, which can weaken the metal and make it moresusceptibletocorrosion.
2) Over-pressure:Theoccurrenceofover-pressureinan ammonia storage tank can have serious safety implications,includingthe risk ofexplosionsorfires. The injection of warm ammonia into the tank or sudden mixing of ammonia solution and liquid ammonia duetothe break upofanoillayercanlead to a rapid increase in pressure beyond the tank's design limits. Proper design, operation, and maintenance of the tank and associated equipment, alongwithappropriatesafetymeasuressuchasrelief valves and emergency shutdown systems, are essential in mitigating the risk of over-pressure and ensuringsafehandlingandstorageofammonia.
3) Overfilling: The accurate monitoring of the liquid level in tanks is critical to ensuring their safe operation. However, errors in level readings by operators,combinedwiththefailureofthehigh-level alarm, can lead to overfilling of the tank. This can result in spills, leaks, or even rupture of the tank, which can pose serious safety hazards to personnel andtheenvironment.
4) Fatigue: Fatigue is a common phenomenon that can occur in materials due to repeated stress cycles over alongperiodoftime.Inthecaseofammoniastorage tanks, fatigue can occur due to the tank's long lifetime,andthestressesthatitexperiencesfromthe constant filling and emptying of the tank, as well as other factors such as temperature changes and corrosion.
5) Faulty design: Human error in the design phase of ammonia storage tanks can result in faulty tank design, which can compromise the safety and integrityofthetank.
6) Implosion: The collapse or implosion of an ammonia storage tank due to vacuum conditions can have serioussafetyconsequences,includingdamagetothe tank, release of ammonia, and injury or death to personnel. This can occur when pressure transmitters fail to accurately detect vacuum conditions in the tank, and the vacuum relief valve fails to open, resulting in the tank's roof partially collapsingduetothenegativepressure.
7) Cooling system failure: Cooling water blockage in an ammonia storage tank can cause the cooling system to fail, leading to overheating and potentially catastrophicconsequences.
2.3 Modelling software

TheAerial LocationsofHazardousAtmospheres(ALOHA) model is a sophisticated computer tool jointly developed by the Environmental Protection Agency (EPA) and the NationalOceanicandAtmosphericAdministration(NOAA) of the United States. It is designed to estimate the movement and dispersion of gases, providing a quick and efficient way to predict the concentration of pollutants in the air downwind from a spill. The model takes into accountawiderangeoffactors,includingthephysicaland toxicological properties of the spilled material, site characteristics, atmospheric conditions, and release circumstances, using both the Gaussian and heavy gas models. With an extensive database containing informationonthousandsofchemicals,ALOHAcanmodel the threat zone for any chemical that may escape from a tankorpipeline.
In this paper, the ALOHA program was applied to assess the potential chemical accidents that could result from ammoniatankreleasesinthefertilizerindustry,including the possible development of such accidents, the resulting threat zones, and their potential impact on the environment.
3. Simulation results and Discussion
Topredictthepotentialhazardsofachemicalspillorleak, One will need to gather a wide range of data - everything from the specific chemical compound involved, to the locationoftheaccident,tothecurrentweatherconditions. All of this information is crucial for accurately modeling andsimulatingthepotentialconsequencesofthechemical dispersion.
Thespecificscenariobeinginvestigatedinthispaperisthe diffusion of gas from a horizontal ammonia tank in the fertilizer industry, which can be caused by any one of the reasons that were mentioned earlier. The dimensions of thedamageareprovided,aswellasthevolumeofthetank andthe percentageto whichit isfilledin the Figure 2. To simulatethepotentialreleaseofgas,themostunfavorable meteorological conditions are taken into account, such as wind speed, temperature, humidity, and atmospheric stability. This information is retrieved from the India meteorologicaldepartment'swebsite.

Finally, the modeling process involves determining the boundaries of zones that could be affected by the hazard, as well as defining a safety zone for people and objects. Thisincludesassessingthepotentialimpactofdemolition, over-pressure of impact waves, and thermal energy effects.
In this study, the researchers investigate the potential dangers of a damaged tank storing ammonia. The tank is pressurizedandkeptataverylowtemperature,andifitis damaged, a significant amount of ammonia gas could be releasedintotheatmosphereinavaporform.Ammoniais bothslightlyflammableandexplosive,whichcouldleadto avarietyofaccidentsscenarios.However,thefocusofthis studyisontherisksassociatedwithfireandexplosion,as these present the greatest danger from the release of ammonia. The study examines various models of how the gascouldspreadandignite,includingthespreadofvapor clouds, jet fires caused by ignition in the cylinder, and liquidexpanding vapor explosions.Theultimategoal is to estimatethepotentialrisksposedtopeopleandobjectsin theplantandsurroundingareas.

Figure 3 illustrates how in each of these scenarios, ammoniawasreleasedfromthestoragetankataspeedof 47 g/s in the form of an aerosol. Ammonia was released foratotalof60seconds.
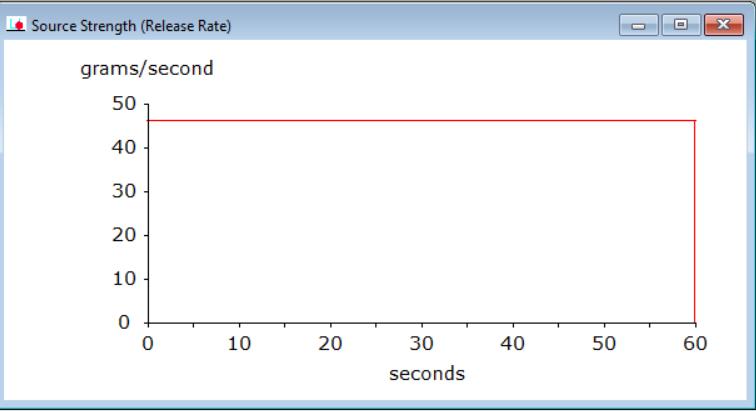
Thefirstscenario,giveninTable2,includesthereleaseof non-burningammoniaformingaflammablevaporcloud.
Ammoniarelease withoutignition withformationof flammablecloud
Temperature 300C
Wind 5mph
Humidity 50%
Stabilityclass B
Cloudcover 5tenths
Ammonia
Upon conducting the chemical accident simulation, the areas with gas vapor air concentration that falls within flammability limits and is prone to starting a fire were identified with precision. These findings are outlined in detail in Figure 4, which depicts the results of the simulation. According to the findings, the gas concentrationintheatmosphereatadistanceof0.2miles from the hazard location spot in the wind direction exceeds 1100 ppm, thereby presenting an extremely high riskoffire(markedinred). Theworkerswhoarepresent at their jobs and the area of the factory nearby are at the greatestriskinthisarea.Theyellowandorangezonesare important areas to consider when assessing the potential hazards associated with the storage and handling of ammonia.Thesezonesextendfrom0.2milesto1.25miles in the wind direction from the source of the hazard. The yellow zone is characterized by a gas concentration of more than 30 parts per million (ppm), while the orange zone has a higher concentration of over 160 ppm. It is essential to take adequate safety measures in these areas to protect personnel and the environment from the
potential dangers posed by ammonia exposure. This may include the use of protective gear and clothing, as well as theimplementationofevacuationproceduresintheevent ofanemergency.
The second simulation estimates the probability of forminganexplosive zonecausedby over-pressure,i.e., it defines the areas on which explosion of the formed ammonia cloud releasing into the atmosphere can occur, eventhoughthesecondscenario,showninTable 3,refers to the simulation of the accident under the same conditions.
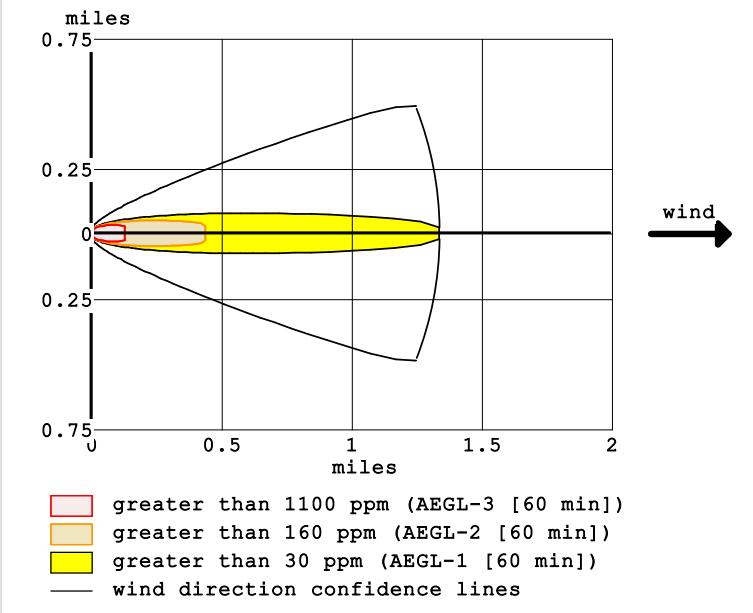
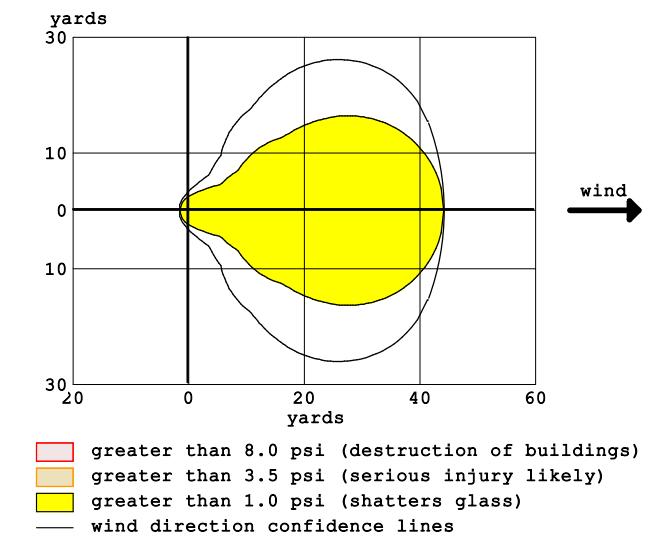
Figure 5 shows that the yellow zone is the area where there is a pressure of more than 1 PSI. In this area, there have been reports of minor human injuries as well as minorstructuralissueslikecrackedwindowsandglasses
Thereleaseofammoniafromaholeinahorizontalstorage tankinflamewaspredictedinthethirdscenario,shownin Table4.
Scenario weather Releasing substance
Ammoniarelease withoutignition withformationof explosivecloud
Wind 5mph
Humidity 50%
Temperature 300C Ammonia
Stabilityclass B
Cloudcover 5tenths
Thesimulation wasperformed for thedetonationignition occurred in the time period of 60 seconds after the gas release.
Wind 5mph
Humidity 50%
Scenario weather Releasing substance Releaseof burningammonia (Jetfire)
Temperature 300C Ammonia
Stabilityclass B
Cloudcover 5tenths
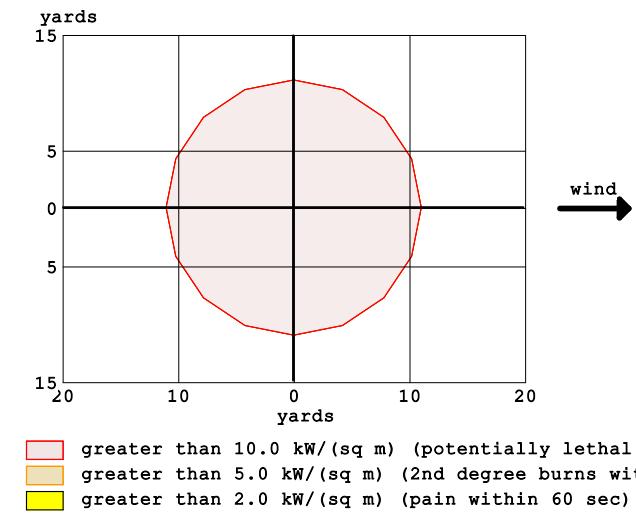
The software warns of a potentially dangerous threat: thermal radiation. This scenario also includes three dangerouszones,asshowninFigure6.
The software predicted a jet flame 11 yards long that would last 60 seconds. Thermal radiation with anenergy greater than 10 kW/m2 is expected in the 11 yard radius red zone. This zone is potentially lethal and can cause severeburnsinaslittleas60seconds.

The fourth scenario, shown in Table 5, was modelled for the Boiling Expanding Vapour Explosion (BLEVE). Thermal radiation (surface heat flux of flame) of the burningtankisthedangerpredictedbythesoftware.
Conditions in Scenario 4
4. CONCLUSIONS
The use of the ALOHA software for accident simulation hasproventobeaneffectivetoolinassessingthepotential consequences of ammonia release from a horizontal storagetankinafertilizerindustry.Thesoftwareprovides a comprehensive analysis of the dispersion and impact of hazardous materials, including fire, explosions, and toxic gas releases. By simulating potential accidents and their consequences, safety professionals can better prepare emergency response plans and mitigate the risks associated with hazardous material storage and transportation.
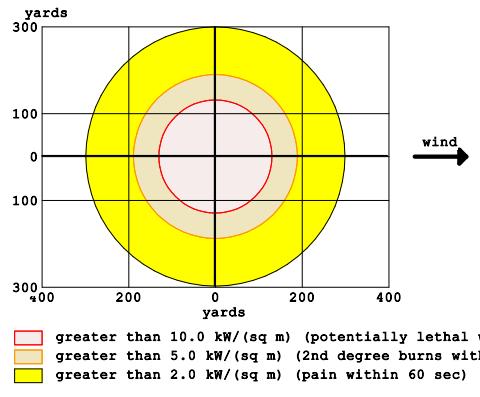
Scenario 4 was created to model the Boiling Expanding Vapour Explosion (BLEVE) and the software predicts the danger of thermal radiation from the burning tank. The characteristicsofthethreatzonesinScenario4areshown inFigure7.ThesoftwarepredictsthatinScenario4,afire with a diameter of 120 yards will occur in just 3 seconds. The redzone, where the fireball originates,hasan energy flux greater than 10 kW/m2 and is potentially fatal to humans. The radius of this zone is almost 120 yards. The orange zone, which occupies the band from 120 to 190 yards, is characterized by a thermal energy of 5 kW/m2 and can cause fires in buildings and serious burns to people. The yellow zone is situated in the subsequent band,whichliesatadistanceof190to300yardsfromthe accident, and the energy of the thermal flux in this band reaches2kW/m2,resultinginminorburns.
The accuracy and reliability of ALOHA have been well established through numerous validations and tests, makingitawidelyrecognizedandacceptedtoolwithinthe safety industry. However, the software is not a substitute forcarefulplanningandadherencetosafetyregulations.It isonly one of manytoolsavailableto safety professionals to help them make informed decisions and manage risks associatedwithhazardousmaterials.
REFERENCES
[1] Khan, F.I, Abbasi, S. 1998. Techniques and methodologies for risk analysis in chemical process industries. Journal of Loss Prevention in the Process Industries, 11(4), pp. 261-277. Discovery Publishing House, New Delhi. doi:10.1016/s09504230(97)00051-x
[2] EL, H.M, Mustapha, S, Choong, T.S.Y, Abdul, R.S, Kadir S.A.S.A, & Abdul, R.Z. 2008. Rapid analysis of risk assessment using developed simulation of chemical industrial accidents software package. International

Journal of Environmental Science & Technology, 5(1), pp.53-64.

[3] Huang, D, Zhang, Q, Li, M, & Liu, M. 2015. Example application of risk assessment technology based on acute poisoning dispersion simulation. In 5th International Conference on Risk Analysis and Crisis Response,RACR;Tangier;Morocco.Pages349-357.
[4] Danijela Ilic Komatina, Jovana Galjak, Svetlana Beslosevic. 2018. Simulation of Chemical Accidents with Acetylene in Messer Tehnogas Kraljevo Plant by Aloha Software Program. University Thought publication in Natural Sciences, VOL 8 NO.2 2018. doi:10.5937/univtho8-18014.
[5] Shanzida Sultana Ema, Anamika Roy, Md Tanvir Sowgath, 2018. Comprehensive Hazard Identification andSafetyEvaluationfor ShahjalalFertilizerIndustry Limited. International Conference on Mechanical, IndustrialandEnergyEngineering2018.
[6] Mannan, S. 2013. Lee’s Process Safety Essentials: Hazard Identification, Assessment and Control. Butterworth-Heinemannbook.
[7] Khan F.1, & Abbasi S.A. 1998. Domiefect: (domino effect) a user-friendly software for domino effect analysis. Environmental Modelling and Software,. 13(2), pp. 163-177. doi: 10.1016/s13648152(98)00018-8.
[8] G.P. Williams, Safety performance in ammonia plants: survey VI, Process Safety Progress, vol 18, pp 78-81, (1999).
[9] ECHA, May. “Guidance on information requirements andchemicalsafetyassessment.”ChapterR8(2008).
[10] J.GMarshall,andJ.H,Burgoyne.TheSizeofflammable clouds arising from continuous releases into the atmosphere, In Inst. Chem. Symp, Vol. 49, P. 103. (1977).
[11] Mohammed Faraj Saeid, Ismail Hassan Abdilahi, Azizan Ramli.2022.Risk assessment of Ammonia Storage Tank facility in a Fertilizer Production Plant BasedonBayesianApproach:ACasestudy.Journalof Global Scientific Research in Chemical Engineering 7(2)/2094-2013.
