A THERMO-ACOSTICAL INVESTIGATION ON AMINO ACETIC ACID IN AQUEOUS KCL SOLUTION
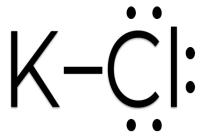 Rina R Bangde*1, Urvashi P. Manik2 and Paritosh L. Mishra3
Rina R Bangde*1, Urvashi P. Manik2 and Paritosh L. Mishra3
1*PG Student, Department of Physics, Sardar Patel Mahavidyalaya, Chandrapur- 442402, Maharashtra, India
2Professor, Department of Physics, Sardar Patel Mahavidyalaya, Chandrapur- 442402, Maharashtra, India
3PGT, Department of Physics, Sardar Patel Mahavidyalaya, Chandrapur- 442402, Maharashtra, India

***
Abstract
In recent years,studiesontheacousticproperties of aqueoussolutionsofmixedelectrolyteshaveprovenuseful in understanding specific ion-ion and ion-solventinteractionsinsolutions. [1] Ultrasonic technique to evaluate the thermodynamic properties of amino acetic acid and proteins. Using speed and density of other thermoacoustic and volumetric.alsocalculatedl,Wada’sconstant(W),Rao’sconstant(R),adiabaticcompressibility(β),acousticimpedance(Z), relativeassociation(RA),Relaxationstrength(r),Surfacetension(σ),SolvationNo,Nonlinearparameter(B/A),Isothermal Compressibility(KT)
Keywords: - AminoAceticAcid,Ultrasonicvelocity,Density,Thermo-acousticalparameters.
Introduction
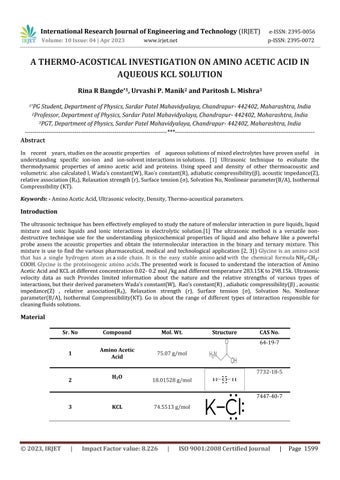
Theultrasonictechniquehasbeeneffectivelyemployedtostudythenatureofmolecularinteractionin pureliquids,liquid mixture and ionic liquids and ionic interactions in electrolytic solution.[1] The ultrasonic method is a versatile nondestructive technique use for the understanding physicochemical properties of liquid and also behave like a powerful probe assess the acoustic properties and obtain the intermolecular interaction in the binary and ternary mixture. This mixture is use to find the various pharmaceutical, medical and technological application [2, 3]) Glycine is an amino acid that has a single hydrogen atom asaside chain. It is the easy stable aminoacidwith the chemical formulaNH₂-CH₂COOH.Glycine is the proteinogenic amino acids The presented work is focused to understand the interaction of Amino AceticAcidandKCLatdifferentconcentration0.02-0.2mol/kganddifferenttemperature283.15Kto298.15k.Ultrasonic velocity data as such Provides limited information about the nature and the relative strengths of various types of interactions,buttheirderivedparametersWada’sconstant(W), Rao’sconstant(R),adiabaticcompressibility(β),acoustic impedance(Z) , relative association(RA), Relaxation strength (r), Surface tension (σ), Solvation No, Nonlinear parameter(B/A), Isothermal Compressibility(KT). Go in about the range of different types of interaction responsible for cleaningfluidssolutions
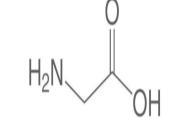
Material Sr. No Compound Mol. Wt. Structure CAS No. 1 Amino Acetic Acid 75.07g/mol 64-19-7 2 H2O 18.01528g/mol 7732-18-5 3 KCL 74.5513g/mol 7447-40-7 International
(IRJET) e-ISSN:2395-0056 Volume: 10 Issue: 04 | Apr 2023 www.irjet.net p-ISSN:2395-0072 © 2023, IRJET | Impact Factor value: 8.226 | ISO 9001:2008 Certified Journal | Page 1599
Research Journal of Engineering and Technology
Methods
TheVelocityanalogultrasonicinterferometer,operatingatafrequencyof2MHz,manufacturedbyMittal Enterprises Pvt.wasusedtomeasureultrasonicvelocity.Ltd., New Delhi(modelF-81) with an accuracyof0.0001m/s.The source oftheultrasonic waves was a quartz crystal excited by ahigh-frequencyoscillator. The cell was filled with the desired solution and constant temperaturewaterwas circulatedthroughthe outershell.The cell was allowed to equilibrate for 30minutes.beforeaction.Thedensitiesofthesolutions. wereaccuratelydeterminedusinga10mlflaskwithadensityof±2*10-2kg/m3 andadigital electronic balance (Contech CA-34)withanaccuracyof±0.0001g. The experimental temperature waskeptconstant by circulatingthewaterusingan automaticthermostaticwaterbathsuppliedbylab-Hosp.AMumbaicompanywithatemperatureaccuracyof±1K[11]. Defining
-
The experimental values of the velocity U and the density р at the temperatures283.15k, 288.15k, 293.15k and 298.15k areshownintable1.Calculatedthevalueofacousticimpedance(Z),adiabaticcompressibility(β),relaxationforce(r),surface tension(σ),relative relationship(Ra),solvation index, Wada’s constant(W), Rao’s constant(R),the isothermal compressibility(KT),thenonlinearparameters(B/A)isshownintable1-4.Thevariabilityofalltheseparameterswiththe concentrationof distilled waterandKCL withaminoaceticacidisshowninfig.1-10.Itisobserved thatthe densityofall the distilled water and aqueous KCL solution increase with the increase in concentration of solution as expected and decrease with increase of temperature due to the thermal energy of the system which distract the intermolecular force.[11]
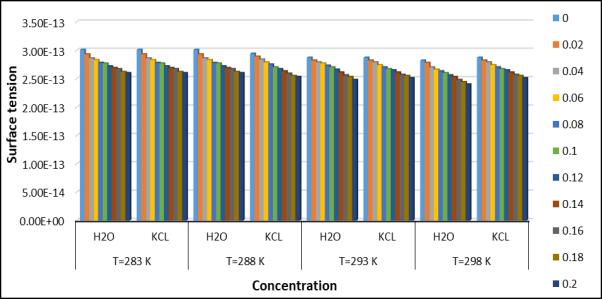
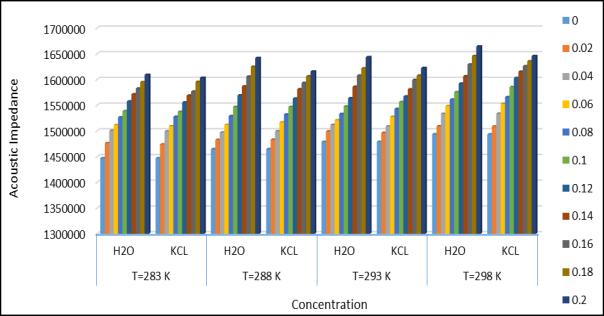
It is observed that the ultrasonic velocity shows the increasing variation with increasing temperature. The ultrasonic velocity shows the increasing variation with increasing temperature. The ultrasonic velocity of water was measured at different temperatures(283.15k,288.15k,293.15k, and 293.15k) and the calculated given in Table1. It is observed that temperatureandconcentrationaffecttheultrasonicwaveintheexistingsystem(AminoAceticAcid+Water+KCL).So,itis showing that the intermolecular interaction with association. It has been observed that the total solution density in an aqueous solution of KCL increasing KCL concentration(if the temperature remains constant) at a constant concentration, the weak electrostatic interaction of the ions does not significantly affect the surface tension. A decrease in acoustic impedance(Z)indicates weak interaction and vice versa. For a KCL solution, Z increases with increasing concentration(temperatureremainsconstant).However,withincreasing temperature(concentrationremainsconstant),Z decreases. The Rao’s and Wada’s Constant remain nearly constant with increasing temperature. May be due to the increase in conductivity of the KCL solution with increasing temperature. Because of this , there is no accumulation of solute molecules in any particular region. However , the two constants above increase with increasing concentration. Relative association(Ra) is a property used to understand interactions. Two important factor influence the relative associations;(1)Degradationofassociatedsolventmoleculesinadditiontothesolute.(2)solvationofaminoacidmolecules. The first involves a decrease and the second an increase in relative association. In the present study, RA increase with aminoacidconcentration,suggestingagreaterinfluenceofthesecondfactorthenthefirst.Theadiabaticcompressibility © 2023, IRJET | Impact Factor value: 8.226 | ISO 9001:2008 Certified Journal | Page 1600

(IRJET) e-ISSN:2395-0056
10 Issue: 04 | Apr 2023 www.irjet.net p-ISSN:2395-0072
International Research Journal of Engineering and Technology
Volume:
Relation:
AcousticalImpedence(Z)=Uр AdiabaticCompressibility(β)= 1 ��2�� RelaxationStrength(r)={1 ( �� ��∞ )2} SurfaceTension(σ)={(6.3∗10 4)���� 3 2} RelativeAssociation(Ra)={(�� ����)(�� ����) 1 3} SolvationNumber=M/M(1-β/β0)(100-x/x) Wada’sconstant(W)=Vmβ-1/7 Rao’sconstant(R)=VmU1/3 IsothermalCompressibility(KT1)={133∗10 8/(64∗10 4�� 3 2��)3/2} IsothermalCompressibility(KT2)={17.1*10-4/T4/9*U2*��1/3} Nonlenearparameter(B/A1)=(2+(0.98*10^4/��) Nonlenearparameter(B/A2)=(-0.5+1.2*104/��) Result and Discussion:
-
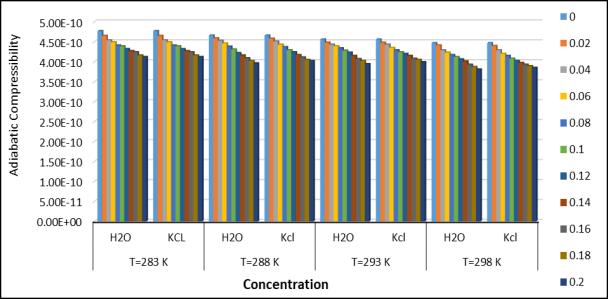
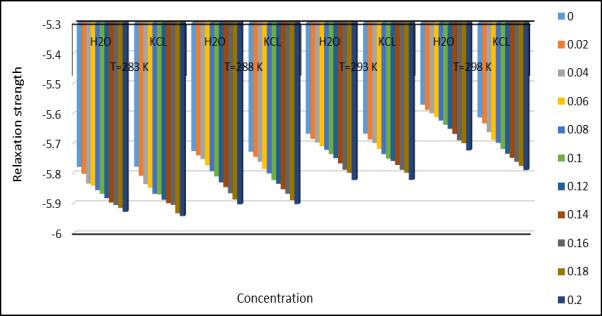
decreaseswithincreasingconcentrationforthefourdifferenttemperatures.Thedecreaseinadiabaticcompressibilitycan be caused by the aggregate of a solvent molecule around a solute molecule. The tested decrease in adiabatic compressibility with concentration of amino acetic acid + KCl + H2O indicates the formation of tightly bound systems[14].Thestrengthof therelaxationdepends entirelyon thefactor[1- UU ∞].Here“U”istheultrasonicvelocityof thesolutionand“U ∞”isaconstantat1600m/s.Adecreasesintherelaxationforcevaluewithincreasingconcentration indicatestheinteractionofthesolutionwiththesolventinthesystem. whichsuggestsalargelinkbetweenfertilizersand saltsalts.Thenonlinearparameter(B/A)obtainedbyHartmann-BalizerandBallousoneisrelatedtotheinternalpressure ,hardness,intermolecularpotential,molecularstructureandmolecularinteraction oftheliquid.Non-linearityparameters for both systems as a function of concentration at 288.15k.(Joshi et al, 2017). The decreasing trends of both parameters indicate that the interaction between the solute and solvent components is stronger at higher concentrations. General trendsinisothermalcompressibility(Kt1andKT2).Itwasfoundthatastheconcentrationafaceticacidincreases,Aminp decreasing fertilizerconcentration appearsto bethe resultsofa correspondingdecrease in free volume.(Millero,1969). Astheweightfractionoffertilizersinelectrolytesolutionsincreases,mostofthewatermoleculesareelectrofiltered,and thereforethewaterdecreases,resultinginadecreaseincompressibility.
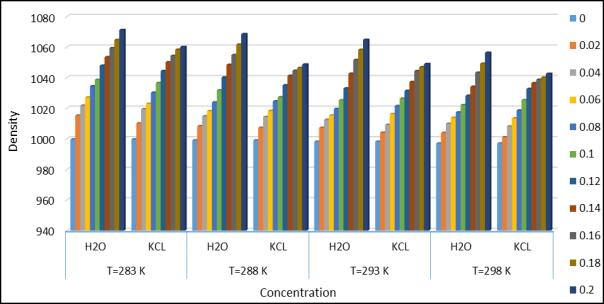
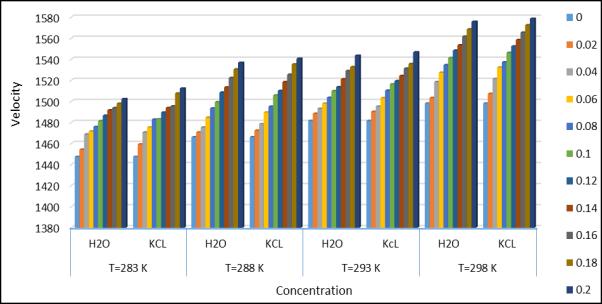

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056 Volume: 10 Issue: 04 | Apr 2023 www.irjet.net p-ISSN:2395-0072
Fig.1.Ultrasonicvelocityatdifferenttemperature andconcentration
Fig.2.UltrasonicDensityatdifferenttemperature andconcentration.
Fig.3.Relaxationstrengthatdifferenttemperature andconcentration
Fig.4.AcousticImpedanceatdifferenttemperature andconcentration
Fig.5.Surfacetensionatdifferenttemperatureand concentration
© 2023, IRJET | Impact Factor value: 8.226 | ISO 9001:2008 Certified Journal | Page 1601
Fig.6.Adiabaticcompressibilityatdifferent temperatureandconcentration
Fig.
Conclusion:
Ultrasoundvelocity and density were measured for both systems with different concentrations of amino acetic acid as solute,at different temperatures (283.15–298.15K). The solvent-solvent interactionpresentindicates that it results in an attractive force due to the structure'stendencyto form, and the solute interaction indicates that it results in an electrostatic force due to the structure'stendencytobeinhibited. According to concentration the value of Velocity, Density, Acoustic impedance are showing the increasing trends and the values of surface tension, adiabatic compressibility, isothermal compressibility, isothermal compressibility, non-linear parameters are showing the decreasingtrends,whichexhibitsagoodassociationintheSystemIIinsteadofSystemI.
Reference
1. Siddique,JamalAkhter NaqviSaeeda,“UltrasonicStudyofBasicα-AminoAcidsinDifferentAcetateSaltSolutions atDifferentTemperatures”.“ChineseJournalofChemistry,”2011,29,669 678
2. ManojKumarPrahara,SarmisthaMishra,“UltrasonicandIonicStudyofAqueousKCLthroughWaldenPlot”,“2020, IRJET”,2020,07Issue:01

3. P.L.Mishra,A.B.Lad,U.P.Manik,journalofScientificresearch,vol.65,June(2021),pp.72-78
4. B.Swain,R.N.MishraandV.N.Dash,Internationaljournalofpureandappliedphysics,vol.13,Jan(2017),pp.4558.
5. M.S.Wagh,R.M.Naktode,Internationaljournalofcurrentengineeringandscientificresearch,vol.6,Jan.(2019),pp. 786-795.
6. S.Pattnaik,U.N.Dash,internationaljournalofpharmaceuticalsciencereviewandresearch,vol.26(2),Jun(2014), pp.201-204
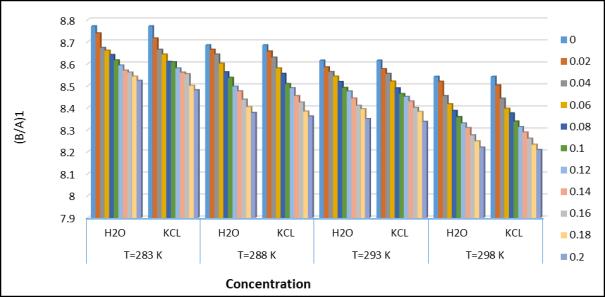 Fig.7Nonlinearparameter(B/A)1atdifferent temperatureandconcentration
Fig.8.Nonlinearparameter(B/A)2atdifferent temperatureandconcentration
Fig.9IsothermalCompressibility(kT1)atdifferent temperatureandconcentration
10IsothermalCompressibility(kT2)at differenttemperatureandconcentration
Fig.7Nonlinearparameter(B/A)1atdifferent temperatureandconcentration
Fig.8.Nonlinearparameter(B/A)2atdifferent temperatureandconcentration
Fig.9IsothermalCompressibility(kT1)atdifferent temperatureandconcentration
10IsothermalCompressibility(kT2)at differenttemperatureandconcentration
6.6 6.8 7 7.2 7.4 7.6 7.8 H2O KCL H2O KCL H2O KCL H2O KCL T=283K T=288K T=293K T=298K (B/A)2 Concentration 0 0.02 0.04 0.06 0.08 0.1 0.12 0.14 0.16 0.18 0.2 H2O KCL H2O KCL H2O KCL H2O kcl T=283 K T=288 K T=293 K T=298 K kT 1 Concentration 0 0.02 0.04 0.06 0.08 0.1 0.12 0.14 0.16 0.18 0.2 H2O KCL H2O KCL H2O KCL H2O kcl T=283 K T=288 K T=293 K T=298 K kT 2 Concentration 0 0.02 0.04 0.06 0.08 0.1 0.12 0.14 0.16 0.18 0.2 © 2023, IRJET | Impact Factor value: 8.226 | ISO 9001:2008 Certified Journal | Page 1602 International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056 Volume: 10 Issue: 04 | Apr 2023 www.irjet.net p-ISSN:2395-0072
7. Siddique,JamalAkhter NaqviSaeeda,“UltrasonicStudyofBasicα-AminoAcidsinDifferentAcetateSaltSolutions atDifferentTemperatures”.“ChineseJournalofChemistry,”2011,29,669 678
8. ManojKumarPrahara,SarmisthaMishra,“UltrasonicandIonicStudyofAqueousKCLthroughWaldenPlot”,“2020, IRJET”,2020,07Issue:01

9. S.C.Patrange,P.R.Sonune,U.P.Manik,P.L.Mishra,RAjournalofappliedresearch,vol.8,May(2022),pp.389-392.
10. S.Punita,R.Uvarani,A.P.Pannerselvam,Internationaljournalofchem.Tech.Research,vol.7,February(2015),pp. 629-638.
11. S.Tiwari,B.S.Kusmariya,A.Tiwari,V.Pathak,A.P.Mishra,journalofTaibah universityfor science,vol.15,Oct (2015),pp.1-26.
12. V.A.Giratkar,R.B.Lanjewar,S.M.Gadegone,InternationaljournalofresearchinBio-Science,Agriculture,and technology,vol.5,September(2017),pp.41-45
13. Y.Akhtar,S.F.Ibrahim,Arebianjournalofresearch,vol.4,October2011,p.487-490
14. Siddique,JamalAkhter NaqviSaeeda,“UltrasonicStudyofBasicα-AminoAcidsinDifferentAcetateSaltSolutions atDifferentTemperatures”.“ChineseJournalofChemistry,”2011,29,669 678
15. ManojKumar Prahara,Sarmistha Mishra,“Ultrasonic andIonicStudy of Aqueous KCL through Walden Plot”, “2020, IRJET”,2020,07Issue:01
Table 1.TheValuesofUltransonicVelocity,Density,Acousticimpedance,AdiabaticCompressibilityatdifferent temperatureandconcentration.
Conc. Velocity Density Acoustic Impedance Adiabatic Compressibility H2O KCL H2O KCL H2O KCL H2O KCL 283 K 0 1447.427 1447.428 999.7 999.7 1446992.77 1446993.77 4.77E-10 4.77E10 0.02 1454.25 1459.27 1015.264 1010.2 1476447.67 1474154.55 4.65E-10 4.65E10 0.04 1468.689 1470.56 1021.803 1019.5 1500710.83 1499235.92 4.54E-10 4.54E10 0.06 1471.531 1475.34 1027.014 1023.023 1511282.94 1509306.75 4.49E-10 4.49E10 0.08 1475.804 1482.815 1034.468 1030.263 1526672.01 1527689.43 4.41E-10 4.41E10 0.1 1481.304 1483.12 1038.653 1036.532 1538560.84 1537301.34 4.39E-10 4.39E10 0.12 1486.512 1489.55 1047.892 1044.362 1557704.03 1555629.42 4.32E-10 4.32E10 0.14 1491.739 1493.9 1053.423 1050.123 1571432.17 1568778.75 4.27E-10 4.27E10 0.16 1493.812 1495.4 1059.361 1054.325 1582486.17 1576637.61 4.24E-10 4.24E10 0.18 1497.963 1507.63 1064.701 1058.422 1594882.7 1595708.76 4.16E-10 4.16E10 0.2 1502.304 1512.32 1071.192 1060.137 1609256.03 1603266.39 4.12E-10 4.12E10 288K 0 1466.032 1466.032 999.1 999.1 1464713 1464713 4.66E-10 4.66E-10 0.02 1470.612 1472.33 1008.314 1007.32 1482839 1483107 4.59E-10 4.58E-10 © 2023, IRJET | Impact Factor value: 8.226 | ISO 9001:2008 Certified Journal | Page 1603 International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056 Volume: 10 Issue: 04 | Apr 2023 www.irjet.net p-ISSN:2395-0072

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056 Volume: 10 Issue: 04 | Apr 2023 www.irjet.net p-ISSN:2395-0072
0.04 1475.307 1478.47 1014.836 1014.36 1497195 1499701 4.53E-10 4.51E-10 0.06 1484.667 1489.52 1018.192 1018.354 1511676 1516859 4.46E-10 4.43E-10 0.08 1493.447 1495.13 1023.849 1024.63 1529064 1531955 4.38E-10 4.37E-10 0.1 1499.309 1505.74 1031.734 1027.237 1546888 1546751 4.31E-10 4.29E-10 0.12 1508.438 1510.12 1040.235 1035.123 1569130 1563160 4.22E-10 4.24E-10 0.14 1513.392 1518.46 1048.497 1041.241 1586787 1581083 4.16E-10 4.17E-10 0.16 1522.348 1525.44 1054.823 1044.559 1605808 1593412 4.09E-10 4.11E-10 0.18 1530.436 1535.19 1061.734 1046.468 1624916 1606527 4.02E-10 4.05E-10 0.2 1536.783 1540.66 1068.496 1048.654 1642046 1615619 3.96E-10 4.02E-10 293 K 0 1481.496 1481.496 998.2 998.2 1478829 1478829 4.56E-10 4.56E-10 0.02 1488.361 1490.263 1007.287 1004.123 1499207 1496407 4.48E-10 4.48E-10 0.04 1493.132 1495.137 1012.499 1009.165 1511795 1508840 4.43E-10 4.43E-10 0.06 1497.934 1503.205 1015.301 1016.25 1520854 1527632 4.39E-10 4.35E-10 0.08 1503.589 1510.33 1019.63 1021.56 1533104 1542893 4.34E-10 4.29E-10 0.1 1509.838 1516.338 1025.213 1026.32 1547906 1556248 4.28E-10 4.24E-10 0.12 1513.612 1519.345 1033.099 1031.45 1563711 1567128 4.23E-10 4.20E-10 0.14 1521.122 1524.258 1042.649 1037.254 1585996 1581043 4.15E-10 4.15E-10 0.16 1528.904 1531.265 1051.643 1044.38 1607861 1599223 4.07E-10 4.08E-10 0.18 1532.634 1535.695 1058.29 1046.929 1621971 1607764 4.02E-10 4.05E-10 0.2 1543.496 1546.65 1064.83 1048.966 1643561 1622383 3.94E-10 3.99E-10 298 K 0 1498.101 1498.101 997 997 1493607 1493607 4.47E-10 4.47E-10 0.02 1503.304 1507.235 1003.936 1001.12 1509221 1508923 4.41E-10 4.40E-10 0.04 1518.44 1521.342 1009.938 1008.19 1533530 1533802 4.29E-10 4.29E-10 0.06 1527.347 1532.214 1013.832 1013.45 1548473 1552822 4.23E-10 4.20E-10 0.08 1534.463 1537.234 1017.36 1018.632 1561101 1565876 4.17E-10 4.15E-10 0.1 1541.321 1546.253 1022.154 1025.32 1575467 1585404 4.12E-10 4.08E-10 0.12 1548.361 1552.342 1028.23 1032.72 1592071 1603135 4.06E-10 4.02E-10 0.14 1553.401 1558.412 1034.117 1036.511 1606398 1615311 4.01E-10 3.97E-10 0.16 1561.63 1565.426 1043.368 1038.697 1629355 1626003 3.93E-10 3.93E-10 0.18 1568.53 1572.426 1049.239 1040.146 1645763 1635553 3.87E-10 3.89E-10 0.2 1575.632 1578.455 1056.345 1042.579 1664411 1645664 3.81E-10 3.85E-10 Conc. Relaxation Strength Surface tension Relative association Solvation Number H2O KCL H2O KCL H2O KCL H2O KCL 283 K 0 -5.77903 -5.77864 3.01E13 3.01E13 0.33333 0.33333 9.18E09 1.26E-10 0.02 -5.80224 -5.80902 2.93E13 2.93E13 0.33693 0.33410 9.16E09 1.26E-10 0.04 -5.83476 -5.83661 2.86E13 2.86E13 0.33577 0.33458 9.13E09 1.25E-10 0.06 -5.84201 -5.84893 2.83E13 2.83E13 0.33683 0.33465 9.12E09 1.25E-10 0.08 -5.85651 -5.86859 2.78E13 2.78E13 0.33829 0.33532 9.11E09 1.25E-10 0.1 -5.86863 -5.87092 2.77E13 2.77E13 0.33840 0.33729 9.10E09 1.25E-10 0.12 -5.88351 -5.88869 2.72E- 2.72E- 0.34021 0.33837 9.09E- 1.25E-10 © 2023, IRJET | Impact Factor value: 8.226 | ISO 9001:2008 Certified Journal | Page 1604
Table 2 Thevaluesofrelaxationstrength,surfacetension,relativeassociation,solvationnoatdifferenttemperatureand concentration.

13 13 09 0.14 -5.89840 -5.90027 2.69E13 2.69E13 0.34081 0.33925 9.08E09 1.24E-10 0.16 -5.90605 -5.90562 2.67E13 2.67E13 0.34225 0.34026 9.07E09 1.24E-10 0.18 -5.91621 -5.93383 2.62E13 2.62E13 0.34303 0.33882 9.07E09 1.24E-10 0.2 -5.92902 -5.94331 2.60E13 2.60E13 0.34309 0.33831 9.06E09 1.24E-10 288K 0 -5.72659 -5.72887 3.01E13 2.94E-13 0.33333 0.33333 1.26E10 1.26E-10 0.02 -5.74079 -5.74538 2.93E13 2.89E-13 0.33536 0.33463 1.26E10 1.26E-10 0.04 -5.7527 -5.76205 2.86E13 2.84E-13 0.33645 0.33557 1.26E10 1.26E-10 0.06 -5.77404 -5.78599 2.83E13 2.79E-13 0.33543 0.33440 1.25E10 1.25E-10 0.08 -5.79345 -5.80075 2.78E13 2.75E-13 0.33532 0.33519 1.25E10 1.25E-10 0.1 -5.81082 -5.82294 2.77E13 2.70E-13 0.33658 0.33368 1.25E10 1.25E-10 0.12 -5.83096 -5.83573 2.72E13 2.67E-13 0.33730 0.33526 1.25E10 1.25E-10 0.14 -5.84635 -5.85363 2.69E13 2.63E-13 0.33886 0.33539 1.25E10 1.25E-10 0.16 -5.86697 -5.8691 2.67E13 2.59E-13 0.33890 0.33492 1.25E10 1.25E-10 0.18 -5.88797 -5.88993 2.62E13 2.55E-13 0.33932 0.33340 1.25E10 1.25E-10 0.2 -5.90402 -5.90317 2.60E13 2.53E-13 0.34007 0.33291 1.25E10 1.25E-10 293 K 0 -5.66833 -5.66833 2.87E13 2.87E-13 0.33333 3 0.33333 3 1.26E10 1.26E-10 0.02 -5.68559 -5.68743 2.82E13 2.82E-13 0.33481 6 0.33333 9 1.26E10 1.26E-10 0.04 -5.69777 -5.69962 2.79E13 2.79E-13 0.33547 3 0.33392 1.26E10 1.26E-10 0.06 -5.7099 -5.71897 2.77E13 2.74E-13 0.33532 3 0.33446 1.26E10 1.26E-10 0.08 -5.72214 -5.73634 2.73E13 2.70E-13 0.33548 7 0.33462 1 1.25E10 1.25E-10 0.1 -5.7365 -5.75176 2.70E13 2.67E-13 0.33592 7 0.33484 9 1.25E10 1.25E-10 0.12 -5.75002 -5.7591 2.66E13 2.65E-13 0.33766 7 0.33585 6 1.25E10 1.25E-10 0.14 -5.76775 -5.77263 2.61E13 2.61E-13 0.33910 6 0.33665 8 1.25E10 1.25E-10 0.16 -5.78864 -5.78832 2.56E13 2.57E-13 0.34029 0.33741 9 1.25E10 1.25E-10 0.18 -5.79922 -5.79874 2.53E13 2.55E-13 0.34160 8 0.33726 7 1.25E10 1.25E-10 0.2 -5.82333 -5.82266 2.48E13 2.51E-13 0.3413 0.33553 1.25E10 1.25E-10 298 K 0 -5.57195 -5.61403 2.82E- 2.87E-13 0.33333 0.33333 1.26E- 1.26E-10 International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056 Volume: 10 Issue: 04 | Apr 2023 www.irjet.net p-ISSN:2395-0072 © 2023, IRJET | Impact Factor value: 8.226 | ISO 9001:2008 Certified Journal | Page 1605

International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056 Volume: 10 Issue: 04 | Apr 2023 www.irjet.net p-ISSN:2395-0072
13 3 3 10 0.02 -5.58882 -5.63399 2.78E13 2.82E-13 0.33449 1 0.33268 2 1.26E10 1.26E-10 0.04 -5.60084 -5.66324 2.70E13 2.79E-13 0.33313 6 0.33192 5 1.26E10 1.26E-10 0.06 -5.61281 -5.68866 2.66E13 2.74E-13 0.33247 0.33128 9 1.26E10 1.26E-10 0.08 -5.62482 -5.69902 2.63E13 2.70E-13 0.33208 0.33189 6 1.26E10 1.26E-10 0.1 -5.63891 -5.71967 2.60E13 2.67E-13 0.33216 0.33212 7 1.26E10 1.26E-10 0.12 -5.65213 -5.73536 2.56E13 2.65E-13 0.33261 6 0.33321 1 1.26E10 1.26E-10 0.14 -5.6694 -5.74892 2.53E13 2.61E-13 0.33343 5 0.33313 2 1.26E10 1.26E-10 0.16 -5.68996 -5.76245 2.48E13 2.57E-13 0.33464 5 0.33233 9 1.26E10 1.26E-10 0.18 -5.70034 -5.77602 2.44E13 2.55E-13 0.33504 7 0.33132 1 1.24E10 1.26E-10 0.2 -5.72407 -5.7897 2.40E13 2.51E-13 0.33579 6 0.33082 7 1.24E10 1.26E-10 Conc. KT1 KT2 B/A1 B/A2 H2O KCL H2O KCL H2O KCL H2O KCL 283 K 0 6.36E-11 6.64E-11 6.36E-11 6.64E-11 8.770635 8.770630 7.79057 7.79056 0.02 6.1E-11 6.5E-11 6.15E-11 6.51E-11 8.738868 8.715686 7.75167 7.72328 0.04 5.94E-11 6.38E-11 5.96E-11 6.39E-11 8.672617 8.664128 7.67055 7.66015 0.06 5.85E-11 6.33E-11 5.88E-11 6.34E-11 8.659730 8.642536 7.65477 7.63371 0.08 5.72E-11 6.25E-11 5.76E-11 6.26E-11 8.640448 8.609051 7.63116 7.59271 0.1 5.68E-11 6.24E-11 5.7E-11 6.25E-11 8.615792 8.607691 7.60097 7.59105 0.12 5.56E-11 6.17E-11 5.58E-11 6.18E-11 8.592614 8.579168 7.57258 7.55612 0.14 5.48E-11 6.12E-11 5.5E-11 6.13E-11 8.569513 8.560010 7.54430 7.53266 0.16 5.42E-11 6.1E-11 5.46E-11 6.11E-11 8.560397 8.553430 7.53313 7.52460 0.18 5.28E-11 5.99E-11 5.33E-11 6E-11 8.542217 8.500268 7.51087 7.45951 0.2 5.19E-11 5.94E-11 5.28E-11 5.96E-11 8.523313 8.480110 7.48773 7.43482 288K 0 6.36E-11 6.3597E-11 6.18E-11 6.4109E-11 8.684711 8.684711 7.68536 7.68536 0.02 6.1E-11 6.1011E-11 6.03E-11 6.3341E-11 8.663892 8.656116 7.65986 7.65034 0.04 5.94E-11 5.9387E-11 5.89E-11 6.2624E-11 8.642685 8.628474 7.63390 7.61649 0.06 5.85E-11 5.8507E-11 5.76E-11 6.1628E-11 8.600807 8.579301 7.58262 7.55628 0.08 5.72E-11 5.7222E-11 5.65E-11 6.1022E-11 8.562001 8.554614 7.53510 7.52605 0.1 5.68E-11 5.685E-11 5.51E-11 6.0044E-11 8.536344 8.508428 7.50368 7.46950 0.12 5.56E-11 5.5556E-11 5.41E-11 5.9547E-11 8.496787 8.48955 7.45524 7.44638 0.14 5.48E-11 5.4759E-11 5.3E-11 5.8786E-11 8.47552 8.453907 7.42920 7.40274 0.16 5.42E-11 5.4177E-11 5.22E-11 5.8172E-11 8.437424 8.424376 7.38256 7.36658 0.18 5.28E-11 5.2793E-11 5.11E-11 5.7361E-11 8.403404 8.383575 7.34090 7.31662 0.2 5.19E-11 5.195E-11 5.06E-11 5.6924E-11 8.376958 8.360910 7.30851 7.28887 © 2023, IRJET | Impact Factor value: 8.226 | ISO 9001:2008 Certified Journal | Page 1606
Table 3.ThevaluesofIsothermalCompressibility,nonlinearparameteratdifferenttemperatureandConcentration

293 K 0 6.36E-11 6.36E-11 6.04E-11 6.24E-11 8.614935 8.614935 7.599921 7.599921 0.02 6.1E-11 6.1E-11 5.86E-11 6.14E-11 8.584424 8.576021 7.56256 7.552270 0.04 5.94E-11 5.94E-11 5.74E-11 6.09E-11 8.563385 8.554583 7.536798 7.526020 0.06 5.85E-11 5.85E-11 5.64E-11 6.01E-11 8.542344 8.519404 7.511034 7.482943 0.08 5.72E-11 5.72E-11 5.52E-11 5.94E-11 8.517739 8.488648 7.480904 7.445284 0.1 5.68E-11 5.68E-11 5.43E-11 5.88E-11 8.490763 8.462939 7.447873 7.413803 0.12 5.56E-11 5.56E-11 5.34E-11 5.85E-11 8.474579 8.450148 7.428056 7.398141 0.14 5.48E-11 5.48E-11 5.26E-11 5.8E-11 8.442613 8.429358 7.388914 7.372683 0.16 5.42E-11 5.42E-11 5.17E-11 5.74E-11 8.40982 8.399937 7.34876 7.336658 0.18 5.28E-11 5.28E-11 5.11E-11 5.7E-11 8.394221 8.381476 7.329658 7.314052 0.2 5.19E-11 5.19E-11 5.02E-11 5.61E-11 8.349223 8.336275 7.274559 7.258704 298 K 0 6.36E-11 6.3597E-11 5.89E-11 6.3597E-11 8.541615 8.541615 7.510141 7.510141 0.02 6.1E-11 6.1011E-11 5.72E-11 6.1011E-11 8.518974 8.501972 7.482417 7.461599 0.04 5.94E-11 5.9387E-11 5.52E-11 5.9387E-11 8.453992 8.441681 7.402848 7.387773 0.06 5.85E-11 5.8507E-11 5.4E-11 5.8507E-11 8.416355 8.395973 7.356761 7.331804 0.08 5.72E-11 5.7222E-11 5.31E-11 5.7222E-11 8.386599 8.375087 7.320325 7.306229 0.1 5.68E-11 5.685E-11 5.19E-11 5.685E-11 8.358182 8.337902 7.285529 7.260696 0.12 5.56E-11 5.5556E-11 5.09E-11 5.5556E-11 8.329273 8.313042 7.250131 7.230255 0.14 5.48E-11 5.4759E-11 5E-11 5.4759E-11 8.308738 8.288453 7.224985 7.200146 0.16 5.42E-11 5.4177E-11 4.92E-11 5.4177E-11 8.275494 8.260277 7.184279 7.165645 0.18 5.28E-11 5.2793E-11 4.85E-11 5.2793E-11 8.247888 8.232408 7.150475 7.131520 0.2 5.19E-11 5.195E-11 4.79E-11 5.195E-11 8.219726 8.208603 7.115992 7.102371 © 2023, IRJET | Impact Factor value: 8.226 | ISO 9001:2008 Certified Journal | Page 1607 International Research Journal of Engineering and Technology (IRJET) e-ISSN:2395-0056 Volume: 10 Issue: 04 | Apr 2023 www.irjet.net p-ISSN:2395-0072

 Rina R Bangde*1, Urvashi P. Manik2 and Paritosh L. Mishra3
Rina R Bangde*1, Urvashi P. Manik2 and Paritosh L. Mishra3












 Fig.7Nonlinearparameter(B/A)1atdifferent temperatureandconcentration
Fig.8.Nonlinearparameter(B/A)2atdifferent temperatureandconcentration
Fig.9IsothermalCompressibility(kT1)atdifferent temperatureandconcentration
10IsothermalCompressibility(kT2)at differenttemperatureandconcentration
Fig.7Nonlinearparameter(B/A)1atdifferent temperatureandconcentration
Fig.8.Nonlinearparameter(B/A)2atdifferent temperatureandconcentration
Fig.9IsothermalCompressibility(kT1)atdifferent temperatureandconcentration
10IsothermalCompressibility(kT2)at differenttemperatureandconcentration