NITRATE ELIMINATION FROM GROUNDWATER WITH THE UTILIZATION OF ACALYPHA INDICA -A REVIEW
R.Vidhya4 and M.Yasvanthini5Assistant Professor, Department of Civil Engineering, Vivekananda College of Technology for women, Tiruchengode, India.
2,3,4,5 UG Student, Department of Civil Engineering, Vivekananda College of Technology for women, Tiruchengode, India
Abstract - Inadequatetreatmentofgroundwaterbefore consumption can be harmful to human health and the environment. We use a lot of water and produce a lot of trash, both of which can be harmful since they include various microorganisms, inorganic substances, and organic compounds. Unsafe ground water effluent results from a variety of physiochemical processes. The soil and water are degraded when chemically polluted ground water mixes with these natural resources and the ecosystems they rely on. The goal of this research is to identifythemostefficientstrategyforpurgingtoxinsfrom underground water supplies. Effluent guidelines and laws for wastewater treatment plants have been implemented by a number of protection authorities across the world basedonperformanceandcontroltechnologies.Thereare three phases of TreatedWaste Water (TWW). TWW treatment that can be distinguished from one another. In the past, TWW removal from water supplies was accomplished using adsorption, flotation, ozone, ion exchange,andcrystallization.Nolongerarethesemethods often used. Water from the ground can be gathered and possibly reused in manufacturing processes utilizing cutting-edge wastewater treatment techniques. This review artlicle provides a literature overview on the commonandactualfeaturesofgroundwater,includingits ingredients such the chemicals used to create simulated ground water with dust and the treatment techniques used to deal with the effluents. This evaluation examines the literature to determine the most efficient absorbent methodfordetoxifyinggroundwaterofnitrates.Activated carbon derivedfromAcalypha indica,itisfound,isa very effectiveabsorbent.
Key words: Ground water, Effluent, TreatedWaste Water (TWW),Activatedcarbonand Acalyphaindica

1. INTRODUCTION
Ground water, nitrogen (N), nitrate (NO3), and nitrite (NO2) are likely to come up in conversations about our project. All known forms of life depend on water as an important chemical element. The oceans on Earth contain the vast majority of the planet's water, which is salty. Thereis3%freshwaterleft.Theicecapandglaciershold
ontoabout68.9%oftheworld'sfreshwater,whileground water holds 30.1%, surface water holds 0.3%, and other forms of fresh water hold 0.9%. Like salt, nitrate is a chemical. Nitrate is ingested with our food and drink. Nitrates in water are typically very low. However, when nitrate levels in water are particularly high, it becomes a major source. Foods like carrots and spinach contain nitrates naturally. The molecule nitrate (NO3) consists of nitrogen and oxygen and is water soluble. It is produced when oxygenated water interacts with nitrogen (from ammoniaorelsewhere).
The rising demand for water in semi-arid regions around the world has increased the urgency with which contaminants, notably dangerous cationic heavy metals and anions, must be eliminated. Since pollution affects so many facets of daily life, it must be considered one of the most pressing issues we face today. Due to population growth,theneedforcleanwaterwillgrowasthepollution problem worsens over the years. As a result, demands on boththeavailabilityandpurityofextractedwaterwillrise [23]. Nitrate anion is a major contributor to water pollution problems. Nitrate occurs naturally as a result of the breakdown of organic nitrogen compounds and is found in low to moderate amounts. The majority of their occurrences in nature were in inorganic materials like rocks and soil. Natural nitrate pollution is also caused by thepresenceofdecayingorganicmatteratgreatdepthsin thesoil[24].
Nitrate is a normal component of plant matter, and its concentration in harvested vegetables varies with the quantity of fertilizer used and other factors. Nitratenitrogenisfoundprimarilyinvegetablesincludinglettuce, celery,beets,andspinach,andtheaverageadultconsumes 20–70 milligrams per day, as reported by the World Health Organization. Nitrates are not toxic when consumed in moderation as part of a healthy diet. Nitrate is produced when oxygenated water interacts with nitrogen from ammonia or another source. Vegetables have different amounts of nitrate due to factors including thetypeandquantityoffertilizerused,amongothers.
1.1 SOURCES OF NITRATE POLLUTION IN GROUND WATER
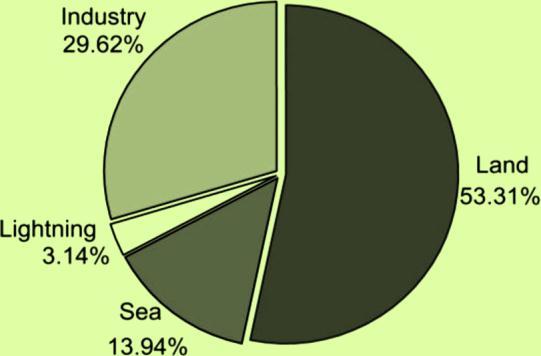

Nitrateconcentrationsingroundwaterincreaseasaresult of human activities like agriculture, industry, home effluents,andemissionsfromcombustionengines,despite naturally low nitrate levels (usually less than 10 mg/l NO3). It takes around 20 years from the time pollution beginsuntilitisdetectedingroundwaterbecausenitrates travel so slowly in soil and groundwater. Nitrate concentrationsareexpectedtoremainaffectedbyexisting polluting operations for several decades. However, if the aquiferpressureishigh,transitwithinthesaturationzone canbequitequick.
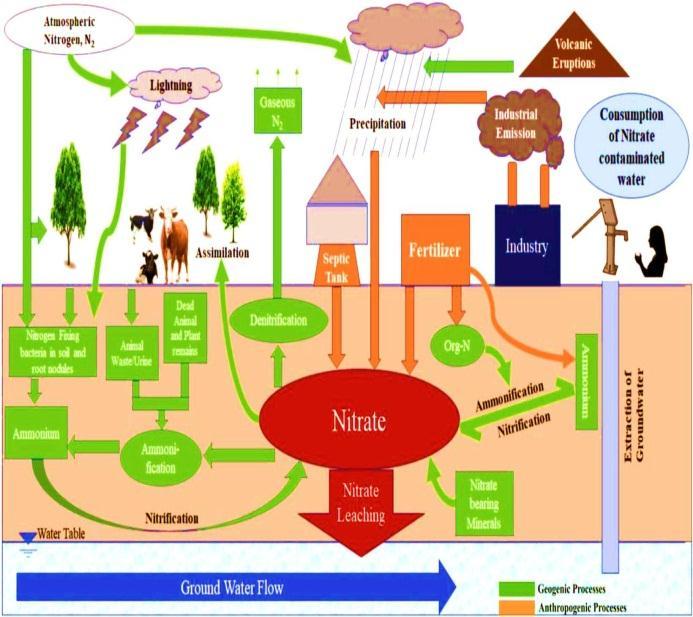
Cultivation in areas with thin soil layers, poor nutrient bufferingcapacity,orwherelandusechanges;
• Over fertilization of crops to increase agricultural activity;
• Widespread cultivation of crops that need a lot of fertilizer and leave the soil bare for long periods of time (maize,tobacco,andvegetables);
•Drainagesystemsthatwashfertilizersaway;
• Intensive agricultural rotation cycles that involve crops thataregrowninthesameplaceyearafteryear.
Among other sources, it's hard to find scattered sources. These sources include organic fertilizer from animal husbandry, ground water pollution from septic and sewage discharges, leakage of industrial corrosion inhibitors into the ecosystem, farming in areas where the soil layer is thin or where land use is changing, overfertilization of crops to increase agricultural activity, and more people living in cities. Most of the water that goes back into aquifers under many towns around the world comes from sewage and water supply systems that leak. Figure1showstheestimatedannual amountsofnitrogen fixedfromdifferentsources.
1.2 HUMAN HEALTH RISK
Overshooting the allowable amount of 100mg/l for nitrates in drinking water has been linked to numerous adverse health effects. The water table is replenished by ground water, which originates deep below the surface of the planet. Nitrate levels were once low, but poisoning of the aquifer has greatly increased them. Nitrate contaminationofdrinkingwaterisincreasingasaresultof the discharge of domestic and industrial wastewater, as well as agricultural fertilizers, into the ground. Nitrate in waterisaleadingcauseofmethemoglobimia,oftenknown as blue baby syndrome, an illness affecting infants in whichtheironintheirhaemoglobinisoxidizedtoa ferric form, rendering the blood cells unable to transport oxygen. Nitrate poisoning is more common in infants less than six months because harmful bacteria thrive in their immaturedigestivesystems.
Mathemoglobin is a molecule formed when nitrite combineswithoxygen-carryinghaemoglobinintheblood. Therefore, the blood's ability to transport oxygen is diminished. Mathemoglobin, a disease caused by a lack of oxygen, manifests itself in neonates in the form of suffocation symptoms. Bluish skin, especially around the eyes and lips, is the most obvious symptom of mathemoglobin. Methylene blue, when injected intravenously, successfully converts mathemoglobin to haemoglobin, allowing for successful treatment. If the patient does not receive therapy, they will die. Only after 70% of haemoglobin is transformed to matheglobin will deathoccur.
Goitre has been linked to increased nitrate consumption, according to certain studies. Nitrate levels in drinking water have been linked to an increased risk of goiter, albeit this link has not been definitively established [25] Nitrates in drinking water have been linked to a higher
risk of having a child born with abnormalities [26]. Neither has this been demonstrated, and studies in animals have failed to find a link (25] Additionally, hypertension non-Hodgkin's lymphoma and higher infant mortalityhaveallbeenlinkedtocigarettesmoking[27,28]
Water having dangerous levels of nitrate should not be utilized as drinking water, as the best course of action is preventionratherthantreatment.Thisisinadditiontothe prudent use of water and fertilizers in agricultural fields. Thiscanbeachievedthroughroutinemonitoringofwater qualitytodeterminenitrateconcentrations.Wellsthatare poorly built or situated may allow nitrate-contaminated groundwatertoseepin,makingthewaterunsafetodrink. Thisisespeciallytrueforunlined,shallowwellsthatallow a lot of percolation from the surrounding region and can become contaminated by floodwaters carrying high levels of nitrate from agricultural fields. Contamination of wells is commonplace due to the proximity of feedlots, barnyards, and septic tank systems. It has been hypothesized that wells deeper than 30 meters(100 feet) are quite secure. Decontaminating water with a high nitrate content using standard in-line filters is inefficient. Deionization, desalination, reverse osmosis, biological denitrification, electrodialysis, etc., are all viable methods forremovingnitratefromwater.
Nitrate toxicity has been linked to a number of other health issues, including but not limited to the following: oral cancer,cancerofthecolon,canceroftherectum,and other gastrointestinal cancers Alzheimer's disease, vascular dementia of the biswanger type or the multiple small in fact type, absorptive and secretive functional disorders of the intestinal mucosa, and changes in maturation, differentiation, and apopto Hypertension, increased infant mortality, goiter, thyroid condition, and birth deformities are all related to excessive nitrate in drinking water. The requirement for vitamin A in the diet may rise if nitrates are included. New evidence suggests that this kind of connection may not be all that useful under typical eating situations. Table 1 lists the concentrations of nitrate in groundwater from various sources.
2. ISSUE OF NITRATES CONTAMINATION IN WATER
Nitrate is naturally present in water below 10mg/l. The activities the recent times have increased nitrate level in the ground water which has exceeded the required limit (45mg/l) as per BIS. This undesired nitrate level in water also causes many nuisances to animals and to other industrial operations. The inconveniences caused by excess nitrate are, 1) Eutrophication is an ecosystem responsetotheadditionofnaturalorartificialsubstances likenitrateandphosphate,throughfertilizerorsewageto aquaticsystemlikelake,pondetc.
During Eutrophication, the 7 increase of phytoplankton takes place so that it leads to hypoxia conditions, where oxygen depletion takes place which induce in decrease in fish and animal population. 2) Indirectly animals ingested withinfectedwillfacesevereproblemsduetotransitionof nitrate to nitrite where haemoglobin where oxygen carryingcapacityisreduced.Insuchcasesanimalundergo death due to deprivation3) Plants with a high nitrate uptake would presumably have nitrate-rich fruit. Without sufficient water, energy from sunlight, and temperature suitabletorapidchemicalreactions,anypersonoranimal consumingsuchfruitorcropwouldconfronttheproblem duetoexcessnitratethroughrootandaccumulatethemin the lower portion of the leaf and stalk. 4) Nitrate is colourless, soluble in water so that its presence can be identified after chemical analysis of water. It can be observedthatthepresence ofnitrate will leadtoaddition of other contaminant to water. This implies that nitrate removal is necessary since nitrate can be used to detect the presence of other ions in water that could potentially disruptthemanufacturingprocess.
The primary function of on-ground nitrogen loading is nitrate contamination in groundwater. However, identifying and differentiating sources is difficult and therefore a framework is needed to narrow down the possibilities. Although Almasri [14] has presented a comprehensive conceptual management framework, it focuses solely on management approaches and avoids addressing the issue of root cause isolation. It is possible that not all research will be able to make use of the framework with mathematical modelling because it is time-consuming,requiresspecialized people, andplacesa premium on careful data collecting (both qualitative and quantitative). Time is of the essence, as many of these areas will already be exploring other potential sources of watersupply.Informationalvariation
2.1 NITRATE ERADICATION METHODS
Nitrate removal from ground water can be accomplished in several ways. Treatment method is selected based on quality of the raw water, extend of treatment given to

water, availability of resource, financial resource availability.
Thefollowingarethemethodstocontrolnitrateinwater,
1.Denitrificationprocess
2.Filtrationtechnique
3.Electrodialysis
2.2 ADSORPTION METHOD FOR ELIMINATION OF NITRATE BY USING ACALYPHA INDICA
Phytostabilization,asdescribed byFritioffandGreger[1], is the process by which plants prevent pollutants from spreading or leaking into the ground or other media by accumulating them in their root zones. The plants were shown to be effective at phytostabilizing Pb rather than phytoextracting it, as evidenced by the larger concentration in the root region observed for both treatmentsandforbothspecies.
The young saplings of Amaranth sps proved the most effective in sequestering Pb, as reported by Ziarati and Alaedini [2], who measured the % Pb accumulation over the course of three harvests and found it to be 32.1%, 33.6%, and 35.1%. Therefore, the data suggest that, despitethefactthatbothplantspecieshavelowTFvalues, A.viridisisabettertranslocatorofPbthanA.thaliana.
AccordingtoReevesandBaker[3],aspeciesisconsidered a Cu hyperaccumulator if its aboveground biomass containsatleast1000mg/kgoftheheavymetal.
Anconaetal.[7]classifiedheavymetalsandfoundPband Cr to be the least bioavailable. This data supports the currentfindingsthatatelevatedlevelsofPbinthesoil,the uptake in the shoot of A. indica increased in the 100% treatment, i.e., 63.87% Pb accumulation in total biomass comparedto32.78%depositioninthe50%treatment.
Zhao and Duo [4] observed in a study that some plants operate as excluders by not allowing pollutants into their metabolic pathways to protect themselves as a selfdefensive mechanism and restricting the concentration in the root zone from exceeding by selectively removing its entry.
Higher TF values in plant species are noticed when high metal concentration is present in the soil, making it available to the root region, and then consecutively translocating a good quantity to the shoot section, as described by El-Mahrouk et al. [8]. For phytoextraction, plantswithmorethanoneTFarepreferable.
According to Ndeda and Manohar [5], the bioconcentration factor (BCF) of a plant determines how much of a pollutant is stored in its tissue relative to its concentration in the medium in which it grows. Similarly, a translocation factor (TF) greater than 1 indicates that the plant has a mechanism for transporting heavy metals from the soil to the plant's aboveground parts, making it anideal candidateforremediatingpollutedlandandlater extractingthemetalsfromtheair.
Gunwal et al. [6] reported that Pb is a heavy metal with low solubility. This means that even at high amounts, it movesslowly,andifitbuildsupintheroot,itmovesvery littletootherpartsofmostplants'bodies.
Rehman et al. [9] collected and studied A. indica growing wild in an industrialized environment, finding Pb concentrationsof2.5mg/kgintheshootand2.9mg/kgin therootzone,withaTFvalueof0.9.Besidestheaffinityof the plant species, the type of metal ion being phytoextracted also plays a role in the translocation from theroottotheshoot.

Acalypha indica was found to be a Pb accumulator with a defensemechanismtodetoxifythePb-inducedtoxiceffect on it by Venkatachalam et al. [10], while very high accumulations of Cd, Cu, and Zn were observed in Amaranthus viridis growing in locally heavily polluted soils.
3. CONCLUSIONS
The study is carried to remove nitrate from drinking water. The efficiency of two different AC prepared from leafandstemfromasameplant,acalyphaindica,aropical weed is made use in study. The potential of different activated carbon (AC) is found with different pH values, adsorbentdosage,concentrationsandtemperature.
Drinkingwateriscontaminatedbynitrateinmanypartsof the world. Because of the complexity of the biogeochemical mechanisms that drive the occurrence of elevated nitrate concentrations in groundwater, determining their origin is a difficult task. Statistics and isotopic analysis have been used by several scientists to determine the origin of nitrate contamination in groundwaterandto distinguishbetweenhumanactivities and natural processes. However, there are certain restrictions to these approaches, and a more thorough methodologyisneedtobeadoptedwheninvestigatingthe processandoriginofleaching.
Furthermore,geogenicaspectsanddetailedstudiesforthe same are somewhat overlooked, despite their relevance. Perhaps isotopic analysis will perform better in determining where nitrate in groundwater comes from, butstatisticalmethodswillbemoreusefulforfiguringout howitgetsthere.Therefore,theproposedframeworkand differentiationtechniquesshouldbeappliedonacase-bycase basis, with a focus on the potential role of geogenic factors in driving the increased nitrate levels. This comprehensive strategy could also be used as a guide for creating new methods to get rid of nitrates in water. Nitrate-contaminated drinking water is more likely to include additional contaminants. The purified, bacteriafreewaterthatremainsinthesupernatantaftertreatment is ideal for consumption. Since Acalypha indica is commonly used for its antibacterial property (Krishna krishna et al., 2010), employing AC for nitrate removal ensures contaminant-free water while also being simple andtoxic-free.
4. REFERENCES
1. FritioffA,GregerM(2003)Aquaticandterrestrial plant species with potential to remove heavy metals from stormwater. Int J Phytorem 5(3), pp.211
224
2. ZiaratiP,AlaediniS(2014)Thephytoremediation technique for cleaning up contaminated soil byAmaranthussp.JEnvironAnalToxicol4(208), 0525–2161
3. ReevesRD,BakerAJM(2000)Metalaccumulating plants. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: using plants to clean up the environment. Wiley, New York, pp 193
230
4. ZhaoS,DuoL (2015)Bioaccumulationof cadium, copper, zinc, and nickel by weed species from municipalsolidwastecompost.PolJEnvironStud 24(1),pp.413–417
5. Ndeda LA, Manohar S (2014) Bio concentration factor and translocation ability of heavy metals within different habitats of hydrophytes in Nairobi Dam, Kenya. J Environ Sci Toxicol Food Technol8(5),pp.42–45
6. Gunwal I, Singh L, Mago P (2014) Comparison of phytoremediation of cadmium and nickel from contaminated soil byVetiveria zizanioidesL. Int J SciResPubl4(10),pp.1–7.
7. Ancona V, Caracciolo AB, Campanale C, Rascio I, Grenni P, Di Lenola M, Bagnuolo G, Uricchio VF (2019)Heavymetalphytoremediationofapoplar
clone in a contaminated soil in southern Italy. J ChemTechnolBiotechnol. https://doi.org/10.1002/jctb.6145

8. El-Mahrouk ESM, Eisa EAH, Hegazi MA, AbdelGayed MES, Dewir YH, El-Mahrouk ME, Naidoo Y (2019) Phytoremediation of cadmium-, copper-, and lead-contaminated soil by Salix mucronata (Synonym Salix safsaf). HortScience 54(7), pp. 1249
1257
9. Rehman MZ, Rizwan M, Ali S, Ok YS, Ishaque W, Nawaz MF, Akmal F, Waqar M (2017) Remediationofheavymetalcontaminatedsoilsby using Solanum nigrum: a review. Ecotoxicol EnvironSaf143,pp.236–248
10. Venkatachalam P, Jayalakshmi N, Geetha N, Sahi SV, Sharma NC, Rene ER, Sarkar SK, Favas PJ (2017) Accumulation efficiency, genotoxicity and antioxidant defense mechanisms in medicinal plantAcalypha indicaL. under lead stress. Chemosphere171,pp.544
553
11. Gardner, W. S., McCarthy, M. J., An, S., Sobolev, D., Sell, K. S. and Brock, D., (2006) Nitrogen fixation and dissimilatory nitrate reduc- tion to ammonium(DNRA)supportnitrogendynamicsin Texas estuaries. Limnol. Oceanogr., 51(1), pp.558–568.
12. Rivett,M.O.,Russ,S.R.,Morgan,P.,Smith,J.W.N. and Bem- ment, C. D., (2008) Nitrate attenuation in groundwater: a review of biogeochemical controlling processes. Water Res.,42, pp. 4215–4232.
13. Holloway, J. M. and Smith, R. L., (2005) Nitrogen and carbon flow from rock to water: Regulation through soil biogeochemical processes, Mokelumne River watershed, California, and Grand Valley, Colo- rado. J. Geophys. Res.,110(F01010);doi:10.1029/2004JF000124.
14. Almasri, M. N., (2007) Nitrate contamination of groundwater: a concep- tual management framework. Environ. Impact Assess., ,27, pp.220
242.
15. Venterea, R. T., Maharjan, B. and Dolan, M. S., (2011) Fertilizer source and tillage effects on yield-scaled nitrous oxide emissions in a corn cropping system. J. Environ. Qual, 40, pp.1521–1531
16. Giammarino,M.andQuatto, P.,(2015)Nitratesin drinkingwater:rela- tionwithintensivelivestock production.J.Prev.Med.Hyg.,56,187–189.
17. Dahan, O., Babad, A., Lazarovitch, N., Russak, E. E. and Kurtzman, D., (2014) Nitrate leaching from intensive organic farms to groundwater. Hydrol. EarthSyst.Sci.,18,pp.333–341.
18. Eiswirth, M., Hötzl, H. and Burn, L. S., (2000) Development scenarios for sustainable urban water systems. In Groundwater: Past AchievementsandFutureChallenges(ed.Sililo,O. etal.),Bal-kema,Rotterdam,pp.917–922.
19. Wang,L.,Ming,Y.,Rios,J.F.,Fernandes,R.,Lee,P. Z. and Hicks, R. W., (2013) Estimation of nitrate load from septic systems to surface water bodies using an ArcGIS-based software. Environ. Earth Sci.,doi:10.1007/s12665-013-2283-5.
20. Wakida, F. T. and Lerner, D. N., (2005) Nonagricultural sources of groundwater nitrate: a reviewandcasestudy.WaterRes.,39,pp.3–16.
21. Feichtinger, F., Smidt, S. and Klaghofer, E., (2002) Water and nitrate fluxes at a forest site in the north Tyrolean Limestone Alps. Envi- ron. Sci. Pollut.Res.,2,31–36.
22. Miller, W. W., Johnson, D. W., Denton, C., Verburg, P. S. J., Dana, G. L. and Walker, R. F., (2005) Inconspicuous nutrient laden sur- face runoff frommatureforestSierranwatersheds.WaterAir SoilPoll.,163,pp.3–17.
23. Rezaee,A.,Godini,H.,Dehestani,S.&Khavanin,A., (2008) Application of impregnated almond shell activated carbon by zinc and zinc sulfate for nitrate removal from water. Journal of Environmental Health Science & Engineering 5 (2),125–130.

24. Hagerty, P. A. & Taylor, J. R. (2020) Nitrate Removal for on-lot Sewage Treatment Systems: The POINTTM System.http://www.taylorgeoservices.com/paper s/point%20system.PDF(accessed26Feb2020).
25. ECETOC (European Chemical Industry Ecology and Toxicology Centre), Nitrate and drinking water,TechnicalReportNo.27,Brussels,(1988)
26. Dorsche, M.M., Scragg, R.K.R., McMichael, A.J., Baghurst, P.A. and Dyer, K.F., (1984) Congenital Malformations and maternal drinking water supplyingruralSouthAustralia:Acasestudy,Am. J.Epidemiol.,119,473-486
27. Malberg, J.W., Savage, E.P. and Osteryoung, J. (1978) Nitrate in drinking water and the early onsetofhypertension,Environ.Pollu.,15,155-16.
28. Weisenberger, D.D. (1991) Potential health consequences of groundwater contamination by nitrates in Nebraska. In Nitrate Contamination : Exposure,consequenceand control (edI.Bogardi and R.D. Kuzella), NATO ASI Ser. G : Ecological Sciences30,SprigerVerlag,Berlin,pp309-315
