Performance and Emission Analysis of Petrol Engine Fueled with Ethanol and Butanol Blended Gasoline Fuel
Hari Charan Chourasia1 , Alka Bani Agrawal21Student, Master of Engineering, Heat Power Engineering
2Professor and HOD, Automobile Engineering, Department of Mechanical Engineering, University Institute of Technology, Rajiv Gandhi Proudyogiki Vishwavidyalaya, Bhopal, Madhya Pradesh, India- 462033 ***
Abstract
Increasing population & continuous growth resulting the increasing number of vehicles and increasing demand of fuel leading the world towards the worst atmospheric conditions i.e. increasing pollution is one of the biggest problem through which most of the countries are struggling without having a significant solution. Increasing pollution & limited reserves for fossil fuel together with carbon emissions regulations have led to producing sustainable fuels made from renewable materials.
In recent years, the focus has been on using bio fuels as alternate energy sources. Blending bio-fuels with gasoline is one of the methods to be considered under the search for a new source of energy. Alcohols are an important category of bio-fuels. Butanol can be an alternative fuel since it is a liquid and has several physical and chemical properties similar to those of gasoline fuels. Butanol don’t have many of the drawback associated with ethanol. Butanol has also a higher molecular weight than ethanol, and therefore, has reduced vapour pressure, lower water solubility, and higher energy density.
Keywords: Alcohol, Ethanol, Butanol, Biofuel, Alternate fuel, Alcohol-Gasoline Blends

1. INTRODUCTION
Today’sworldisintheeraoftechnologywhereanumberoftechnologicaladvancementsarefacilitatingthemodern world. Millions of inventions and their applications are upgraded a number of times and still in continuation by the research and development activities across the world but everything has its dark side as well. Among the enormous advantagesoftechnologytherearealsoanumberoflimitationsthatledtotheworldtofacethecriticalproblemsrelated totechnologyandtheseproblemsifnotmeettothesolution,maybecomeagreatthreattothewellbeingandexistenceofa healthysociety.Oneofthemajorproblemsassociatedwithindustryandautomobilesisenvironmentalpollution.
Increasing pollution & depletion of fossil fuel has led the interests of scholar to study about the reformulate of alternativefuelandaftervariousresearchesafterlate90’sithasfoundthattheblendingofbiofuelcanbethebestsuited solutiontothisproblem.Accordingtopreviousstudy,theuseoffuelalternativesthatcontainingoxygen(oxygenates)was veryimportantastheadditivecanincreasetheperformanceandefficiencyofthefuel.Basedonpreviousstudy,oneofthe best methods to improve the combustion behaviour is by blending base fuel with additives. Alcohol (Methanol, Ethanol, Butanol)-gasoline blends can reduce air pollution and at the same time offers excellent performance of the engine compared to unblended petroleum fuel . The effect of the alcohol gasoline blends on CO emissions for different engine speeds showed that when alcohol percentage increases, the CO concentration decreases. Carbon content in the blended fuelalsoplaysamajorpartontheemissionofCOduetothelowercarboncontent.
Ithasproveninmanyresearchesthatalcoholreducespollutantemissionwhichresultedtheuseofalcoholspecially ethanolforblendingandasanalternativefuel.Astheethanolcameinthepracticeithasfoundthatithasitsadverseeffect i.e. corrosive effect on the engine materials and components due to that it cannot be used without engine modifications. Many government enforcing the regulatory to the automobile companies to manufacture engines with required modifications as they can withstand the corrosive effect of ethanol but still there is a large number of old vehicles are alreadyinpracticewhichcannotbemadecompatibleforethanolbasedfuel.Hencethereisarequirementofanalternative fuelwhichcanbeusedintheexistingengineswithoutmodificationsbecausemodificationandreplacementofsuchahuge number of vehicles or engines is not practically possible. Many researchers have worked in this direction and butanol, whichisalsoanisomerofalcohol,cameintolight.So,thepresentworkisacomparativeandcompetitivestudyofresults tocheck thesuitabilityofbutanol over ethanol inorder tofindanalternativefuel forexistingpopulationofvehiclesthat cannot be modified to use ethanol based fuel. Under this study, experimental work has been done in which different
samplesofethanolandbutanolblendedfuelsuchasE10,E20,E30,B10,B20&B30hasbeentestedonanolder2strokeSI andtheirperformance&emissioncharacteristicshavebeencompared.
2. MATERIALS AND METHODS

Forthisstudy,IOCLmarketedgasolinewasobtainedfromtheCity’sfillingstationandfuelsamplesarepreparedbymixing thealcoholwithgasolinebyvolume percentage.VolumepercentageofEthanolandButanolwaskeptfrom10-30percent by volume. Total six samples of blended fuel were prepared for the study. The ethanol fuel samples prepared by mixing 10%,20%and30%ethanolof99.9%puritywithgasolinebyvolumeandthesesamplesarenamedasE10,E20 andE30 respectively. Similarly, butanol fuel samples prepared by mixing 10%, 20% and 30% n-butanol of 99% purity with gasolinebyvolumeandthesesamplesarenamedasB10,B20,B30respectively.Inordertounderstandthe behaviourof theseblendedfuelfirstofallweneedtounderstandthepropertiesofblendedfuel.
After preparing the fuel samples it has been tested on a single cylinder two stroke petrol engine test rig at IC Engine Laboratory in Central Workshop under the department of Mechanical Engineering of University Institute of Technology, RGPVBhopal(M.P.).

Aschematicarrangementsofvariouscomponentsoftheexperimentalsetupisshowninthefigure2.1. Theimportant mechanicalandelectricalunitsinvolvedinthetestingareasfollows:-
1. TwoStrokePetrolEngine
2. Fuelsupplysystemwithburette
3. EddyCurrentDynamometer
4. Dynamometercontrolunit
5. DigitalTachometer
6. ExhaustGasAnalyserwithprobe.
As shown in the figure the fuel supply is connected to the carburettor of two stroke petrol engine with control valve by virtueofwhichthecontrolledvolumeoffuelissuppliedtotheengine.Theengineshaftsubjectedtoachaindrivewhichis connected to the Eddy current dynamometer. The applied load can be adjusted by a control unit connected with the dynamometer.Theexhaustgasanalyserwithasufficientlylongerprobeassemblyisusedtoexaminetheexhaustfromthe engine.Theactualarrangementofexperimentalisrepresentedinthefollowingfigure.
Afterpreparationoffuelsamples,100ml.petrolisfedtotheburettewhichisusedtostartandruntheengineuntil the 100 ml fuel is consumed. In this idle running engine is lubricated properly and its components attains the working temperature. Afteridlerunning 100 ml fresh gasoline isfilledinthe burette andadjustment of throttleis doneuntil the engineachieves1500RPM whichcanbe measuredusinga digital tachometer.The probeofexhaustgas isput insidethe exhaust manifold for two minutes and the stable reading of exhaust gas analyzer is noted along with the time taken to consume10ml fuelwiththehelpofthestopwatchatzeroloadcondition.
Aftergettingthereadingsatzeroloadtheloadof0.25KWisappliedbyeddycurrentdynamometerwiththehelpof dynamometercontroller.Duetotheapplicationofloadthespeedofenginegoesdownwhichisadjustedbyincreasingthe fuel supply using the throttle until the engine attains the speed of 1500 RPM again. Now again the probe of exhaust gas analyzerisputinsidetheexhaustmanifoldfortwominutesandthestablereadingofexhaustgasanalyzerisnotedalong withthetimetakentoconsume10ml fuelwiththehelpofthestopwatch.
The same procedure is repeated for 0.50 KW, 0.75 KW and 1 KW load and the readings of exhaust gas analyzer and stopwatchisnotedforrespectiveloads.Thetestingprocedureforblendedfuelsamplesisrepeatedinthesamemanneras followedforthetestingofgasolinefuelsampleandthereadingforeachfuelsampleisnotedcarefullyalongwiththefuel consumption timefor 10ml fuel at no loadand increasingloadas0.25 KW, 0.50 KW,0.75 KWand 1.0 KWrespectively. The value of Brake Thermal Efficiencies (BTE) and BSFC for each fuel is calculated using mathematical relations after calculating the properties of blended fuel samples whereas, the values of pollutant emission obtained directly from the readingsofexhaustgasanalyser.
3. RESULTS AND DISCUSSION
As per experimental results obtained for various fuels, the comparison of performance and emission characteristics of Gasoline, E10, E20, E30 & B10, B20, and B30 can be done in order to identify the suitable fuel blend that can be used in existingSIengineswithoutanymodification.
3.1 Comparison of Performance Characteristics: - The comparison of performance characteristics i.e. Brake ThermalEfficiency(BTE)andBrakespecificfuelconsumption(BSFC)isshownonthebasisofexperimentalresultswhich isbrieflydiscussedasfollows.
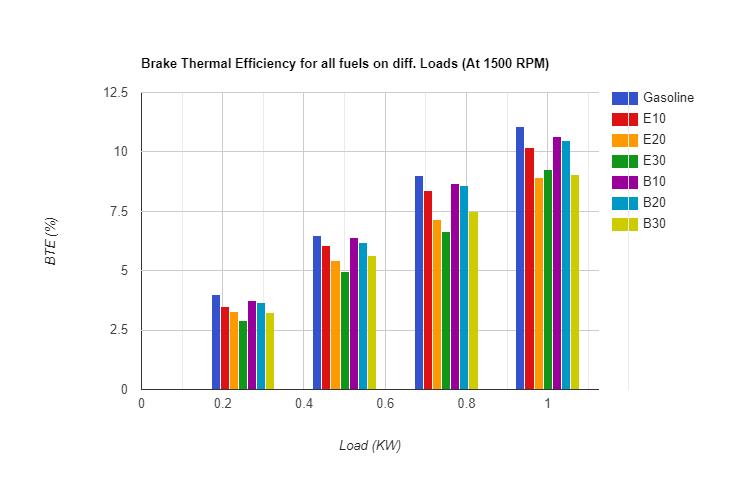
3.1.1 Brake Thermal Efficiency (BTE):- Thecomparisonof brake thermal efficiencyofall fuelsisrepresentedwith thehelpoffollowingfigure

Asshownintheabovefigureitisclearthatbrakethermalefficiencyoffuelblendsislowerascomparedtopuregasoline. Thereasonoflowerbrakethermalefficiencyisnothingbutthelowercalorificvalueofalcoholascomparedtogasoline.As per experimental results, brake thermal efficiency decreases with increasing percentage of alcohol in the blend because alcohol has lower calorific value as compared to gasoline. It can also be seen that brake thermal efficiency of butanol blends is slightly higher than that of ethanol blends because butanol contains comparatively higher energy content per unit volume and lower heat of vaporization as compared to ethanol. Brake Thermal Efficiency is observed as highest for B10amongallfuelblendsduetotheoptimumparametersi.e.balancebetweenlowercalorificvalueandhighervolumetric energycontent.
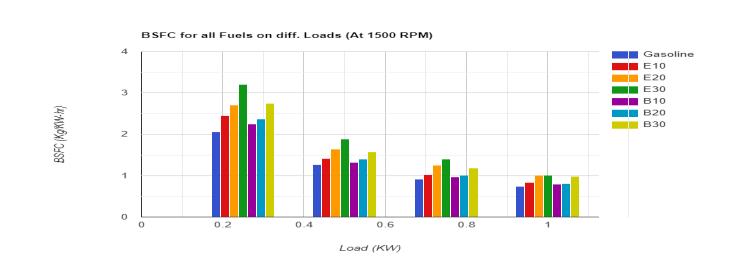
3.1.2 Brake Specific Fuel Consumption (BSFC):-The comparison of brake thermal efficiency of all fuels is representedwiththehelpoffollowingfigure:-
As shown in the above figure it is clear that BSFC for fuel blends is higher as compared to pure gasoline. The reason of higherBSFCisnothingbutthelowercalorificvalueofalcoholascomparedtogasoline. Asperexperimentalresults,BSFC increases with increasing percentage of alcohol in the blend because alcohol has lower calorific value as compared to gasoline. It can also be seen that BSFC of butanol blends is slightly lesser than that of ethanol blends because butanol contains comparatively higher energy content per unit volume and lower heat of vaporization as compared to ethanol. BSFC is observed as lowest for B10 among all fuel blends due to the optimum parameters i.e. balance between lower calorificvalueandhighervolumetricenergycontent.
3.2 Comparison of Emission Characteristics: - The comparison of main pollutants i.e. HC, CO and NOx from the variousfuelsampleisdoneonthebasisofexperimentalresultswhichcanbeunderstandasfollows.
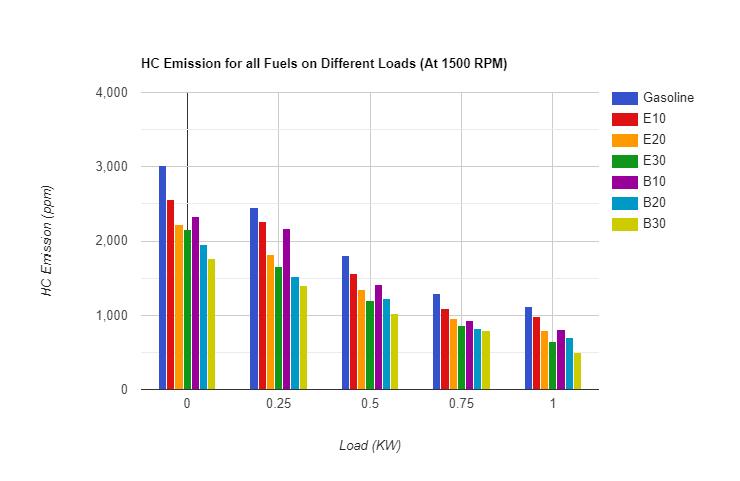
3.2.1 Hydrocarbon (HC) Emission: - As pertheresultsobtainedforunburnthydrocarbonemissionthecomparison ofHCemissionforvariousfuelscanbeunderstandwiththehelpoffollowingfigures.Onthebasisofabovecomparisonthe followingobservationscanbemade:

TheHCEmissionforfuelblendsislowerthanpuregasoline.ThereasonoflowerHCemissionforalcoholfuelblendsisthe presenceofoxygenmoleculesinthealcohol whichaidstothebettercombustionof fuel whereas nooxygenispresentin gasoline.Asthealcoholpercentageincreasesinthefuel,unburnthydrocarbonemissiondecreasesbecausetheamountof oxygen is also increases with alcohol which leans the air fuel mixture and results for better combustion of fuel. HydrocarbonEmissionforButanolblendsisfoundlowerascomparedtoEthanolblends.ThereasonbehindthelesserHC emissionofbutanolisthelowerheatofvaporizationandhighercalorificvalueascomparedtoethanolduetothatbutanol blends burns more efficiently as compared to ethanol blends Hence better combustion results decrease in unburnt hydrocarbonparticles.HydrocarbonEmissionisfoundminimumforB30.Itisbecauseofthehigherpercentageofbutanol whichresultsbettercombustionthanthatofotherfuelblends
3.2.2 Carbon Mono-Oxide (CO) :- As per the results obtained for carbon mono oxide emission, the comparison of COemissionsforvariousfuelscanbeunderstandwiththehelpoffollowingfigures:-
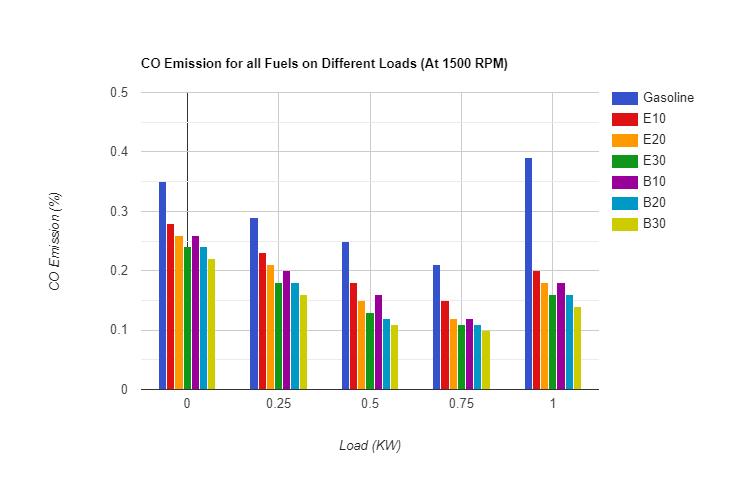

TheCOEmissionforfuelblendsislowerthanpuregasoline.ThereasonoflowerCOemissionforalcoholfuelblendsisthe presenceofoxygenmoleculesinthealcohol whichaidstothebettercombustionof fuel whereasnooxygenispresentin gasoline. Asthealcoholpercentageincreasesinthefuel,COemissiondecreasesuptoacertainvalueofloadduetooxygen richburningandthenincreasesbecauseincreasingloadrequiresmorepowerrapidlybutasalcoholshavecomparatively lowercalorificvalueandhighervapourpressurethangasolinetherefore,gasolinemoleculesburnsfasterinorderto fulfil the energy demandanda slightdelayisobservedinburningof alcohol moleculesascomparedto gasoline whichresults heterogeneousburningoffuelblendandresultincreaseinCOemission.COforbutanolblendsisfoundlowerascompared to Ethanol blends. The reason behind the lesser CO emission of butanol is the lower heat of vaporization and higher calorific value as compared to ethanol due to that butanol blends burns with high temperature as compared to ethanol blends Hence better combustion results decrease in unburnt hydrocarbon particles. Hydrocarbon Emission is found minimumforB30.Itisbecauseofthehigherpercentageofbutanolwhichresultsbettercombustionthanthatofotherfuel blends.
3.2.3 NOx Emission: - AspertheresultsobtainedforunburnthydrocarbonemissionthecomparisonofNOx emission forvariousfuelscanbeunderstandwiththehelpoffollowingfigures.
NOx Emission for all fuel samples at diff. Loads
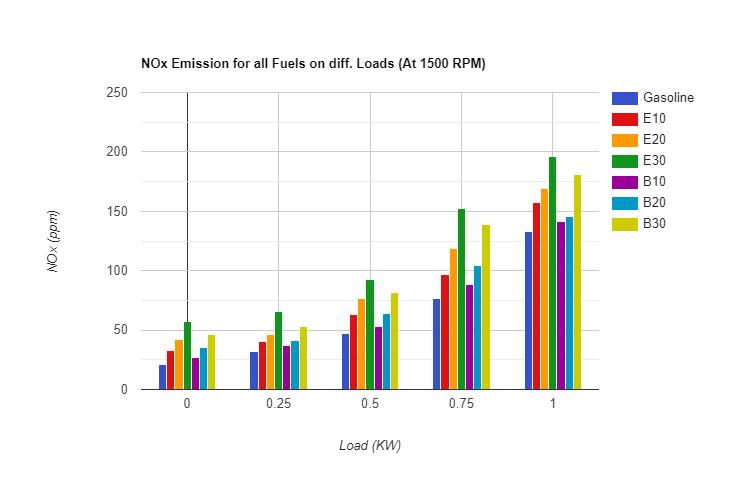
TheNOX Emissionforfuelblendsishigherthanpuregasoline.ThereasonofhigherNOX emissionforalcoholfuelblendsis the presence of oxygen molecules in the alcohol which aids to the better combustion and results increase in cylinder temperatureswhichgivesrisetotheformationofNOX inthecombustionproducts. Asthealcoholpercentageincreasesin the fuel, NOX emission decreases because the amount of oxygen is also increases with alcohol which leans the air fuel mixtureandresultsforbettercombustionoffuelandincreaseofincylindertemperature.NOX Emissionforbutanolblends isfoundlowerascomparedtoEthanolblends.ThereasonbehindthelesserNOX emissionofbutanolistheloweramount of oxygen present in the atomic structure as compared to ethanol. Ethanol consists of 34.78% oxygen whereas butanol consists of 21.62% oxygen by weight due to which ethanol burns with comparatively high temperature than that of butanol and leads to the formation of more NOX in the exhaust. Hydrocarbon Emission is found minimum for B10. It is because of the balance between the higher calorific value and lower amount of oxygen as compared to ethanol blends whichresultstheburningoffuelatoptimumtemperature

4. CONCLUSION:-
On the basis of experimental results obtained it can be concluded that the Brake Thermal Efficiency of fuel blends is slightly lower as compared to the pure gasoline because of the lower calorific value and higher heat of vaporization of alcohols. If we compare individual blended fuel samples, butanol blends shows comparatively higher brake thermal efficiency than ethanol blends which is because of higher calorific value and lower heat of vaporization of butanol as compared to ethanol whereas B10 is the sample identified with maximum efficiency. The Specific fuel consumption is higherforgasoline-alcoholblendsascomparedtopuregasolinewhichisbecauseoflowercalorificvalueandhigherheat of vaporization of alcohols which represent that the fuel consumption will be more for gasoline- alcohol blends as comparedtopuregasoline.IfwecomparetheBSFCofethanolandbutanolblendsitisfoundlowerforbutanolblendsand minimum for B10 due to its higher energy content which represent that butanol blended fuel is more economic than ethanolblendedfuel.
IfwediscussemissioncharacteristicssoithasfoundthattheHCandCOEmissionforfuelblendsislowerthanpure gasoline.ThereasonoflowerHC&COemissionforalcoholfuelblendsisthepresenceofoxygenmoleculesinthealcohol whichaidstothebettercombustionoffuelwhereasnooxygenispresentingasoline.HC&COEmissionforbutanolblends is found lower as compared to Ethanol blends. The reason behind the lesser emission of butanol is the lower heat of vaporization and higher calorific value as compared to ethanol due to that butanol blends burns more efficiently as compared to ethanol blends Hence better combustion results decrease in unburnt hydrocarbon particles. On the other hand the NOX Emission for fuel blends is higher than pure gasoline. The reason of higher NOX emission for alcohol fuel blends is the presence of oxygen molecules in the alcohol which aids to the better combustion and results increase in cylinder temperatures which gives rise to the formation of NOX in the combustion products. NOX Emission for butanol blendsisfoundlowerascomparedtoEthanol blends. Thereason behindthelesserNOX emissionofbutanol is thelower amount of oxygen present in the atomic structure as compared to ethanol. Ethanol consists of 34.78% oxygen whereas butanolconsistsof21.62%oxygenbyweightduetowhichethanolburnswithcomparativelyhightemperaturethanthat ofbutanolandleadstotheformationofmoreNOX intheexhaust.
Theimplementationof butanol blending may bea better alternative toreducepollutantsemissionfrom existing older SI engines while ethanol is suitable for the new generation SI engines or existing engine with modifications. As a large numberofvehiclesarenotpossibletomodifyorreplacewiththenewtherefore,butanolblendingcanplayavitalroleto controltheincreasingpollutionofthefossilfuelsandcanalsosavethepetroleumusingthisgreenfuel.
REFERENCES
[1]Iliev,S.AComparisonofEthanol,Methanol,andButanolBlendingwithGasolineandItsEffectonEnginePerformance andEmissionsUsingEngineSimulation.Processes2021,9,1322.https://doi.org/10.3390/pr90813.
[2]Manish Saraswat1 , AnkurDixit1 ,Abhishek Goel1 and Nathi Ram Chauhan2. Performance and Emission evaluation of ButanolblendsinSIEngine.IOPConf.Series:MaterialsScienceandEngineering691(2019)012081.IOPPublishing doi:10.1088/1757-899X/691/1/012081.
[3]Praveen Kr. Rai1 , Mohd Arif Khan2 , Shubham Awasthi3, To study the Performance and Emissions Characteristics of butanol blended Gasoline Fuel single cylinder Spark Ignition Engine. 3 rd International Conference on “Advances in Power Generation from Renewable Energy Sources” 2019. SSRN-Elsevier at https://hq.ssrn.com/conference=2019APGRES

[4]ShashankSN,SRaviteja&KumarGN.ComparisonofEthanolandn-ButanolBlendswithGasoline:AComputational Study.ISSN:2319–3182,Volume-2,Issue-4,2013.
[5]V. Hönig1,*, M. Kotek2 and J. Mařík2. Use of butanol as a fuel for internal combustion engines. Agronomy Research 12(2),333–340,2014
[6]CristianSandu1*,ConstantinPană1,NiculaeNegurescu1,AlexandruCernat1,CristianNuțu2,andRareșGeorgescu1. Thestudyofthesparkignitionengineoperationatfuellingwithn-butanol-gasolineblends.E3SWebofConferences 180,01010(2020) https://doi.org/10.1051/e3sconf/202018001010
[7]I. Veza* , M. F. M. Said and Z. A. Latiff. Improved Performance, Combustion and Emissions of SI Engine Fuelled with Butanol: A Review. International Journal of Automotive and Mechanical Engineering (IJAME) ISSN: 2229-8649 eISSN:2180-1606VOL.17,ISSUE1,7648–7666DOI:https://doi.org/10.15282/ijame.17.1.2020.13.0568.
[8]F. N. Alasfour, Butanol A Single Cylinder Engine Study: Engine Performance. International Journal of Energy Research,Vol.21,21 30(1997)
[9]Nithin H S, Comparative Study of Performance of Four Stroke Two Wheeler using Ethanol-Gasoline and Butanol Gasoline Blends. International Journal of Engineering Research & Technology (IJERT) ISSN: 2278-0181. www.ijert.org.Vol.4Issue03,March-2015.IJERTV4IS030488.

[10]Magín Lapuerta , Rosario Ballesteros and Javier Barba. Strategies to Introduce n-Butanol in Gasoline Blends. http://www.mdpi.com/journal/sustainability.Sustainability2017,9,589;doi:10.3390/su9040589.
[11]TanTienHuynh1 ,MinhDucLe1 ,DinhNghiaDuong,Efectsofbutanol–gasoline blendsonSIengineperformance,fuel consumption, andemission characteristics atpartial engine speeds. International Journal of Energy and EnvironmentalEngineering(2019)10:483–492https://doi.org/10.1007/s40095-019-0309-9
[12]PreetiNair&H.N.Meenakshi,Reviewonthesynthesis,performanceandtrendsofbutanol:acleanerfueladditivefor gasoline.InternationalJournalofAmbientEnergy.DOI:10.1080/01430750.2021.1873849.
[13]Veloo, PS, Wang, YL, Egolfopoulos, FN, Westbrook, CK,” A comparative experimental and computational study of methanol,ethanol,andn-butanolflames”,CombustionandFlame.157(10),1989–2004(2010).
[14]Tao L., and Aden, A., “The economics of current and future biofuels,” In Vitro Cellular & Developmental Biology –Plant.45(3):pp.199-217,(2009).
[15]M. Koc, Y. Sekmen, T. Topgul, H. S. Yucesu, “The effects of ethanol-unleaded gasoline blends on engine performance andexhaustemissioninasparkignitionengine”,RenewableEnergy,vol.34,pp.2101-2106,(2009).
[16]J.Yang,XLYang,“ExperimentStudyoftheperformanceoftheblendedbutanolgasolinefuel”,SAEC2008A63,(2008).
