Fe-BIOCHAR COMPOSITE FOR THE REMOVAL OF DYE IN WASTEWATER USING ADSORPTION METHOD
1-4UG Students, Department of Civil Engineering, Vignan’s Institute of Information Technology, Visakhapatnam, Andhra Pradesh
***
Abstract - Use of various dyes in order to color the products is a common practice in textile industry. The presence of these dyes in water even at low concentrations is highly visible and undesirable. The biochar composite has become a hotspot as an adsorption material. It is an impurity adsorption material that shows promise and has prospective use in domains related to soil enhancement and sewage purification. This material can effectively remove thedyeinwastewater. Usinga spectrophotometer,kineticstudiesanddyetests,thestructural features and adsorption capabilities are studied. It was found that the biochar composite has a high specific surface area and pore volume. Compared to other adsorbents, this composite truly achieves the concept of “waste control by waste” Food waste derived biochar andFe-Biocharcomposite are prepared by the pyrolysis process. The sequence of addition of ferric chloride differs for food waste derived biochar and Fe-biochar composites and the subsequent removal of Methyl orange dye and Congo red dye from wastewater.
Key Words: Food waste derived biochar, Fe-biochar composite, pyrolysis process, adsorption, muffle furnace, magneticstirrer.
1. INTRODUCTION
Environmental pollution has emerged as one of the unavoidable issues facing the world in recent years as a result of the advancement of industrial growth. The atmosphere, soil, and water are exposed to chemical reagents, biological materials, and certain solid or liquid industrialpollutants.Enormousquantitiesofpollutantsare releasedintotheriver.ParticularlyconsideringinIndia,the increasing number of population led to development of manyindustries.Focusingonthetextileindustry,whichis oneofthemostpollutingindustriesintheworld.20%ofall freshwaterpollutionismadebytextiletreatmentanddying. AtHaridwar,India,researcherstestedthelevelsofmetalsin the soil and groundwater that are close to the textile and tannerybusinesses.Accordingtothefindings,thelevelsof allthemetals,includingchromium,iron,manganese,copper, lead,andcadmium,washigherthanthoseconsideredsafeby theWorldHealthOrganization(WHO).Thesecanleadtoa varietyofissuesforlivingthings.Varioustoxicdyesarealso
releasedbythetextileindustrieswhicharenothingbutthe mainfocusofthispaper.
1.1 Dye-Usage of dye in textile industry

A dye is a material, either natural or artificial, used to colororalterthecolorofsomething.Tochemicallybindthe dye'scolortothefiber,dyeisappliedinanaqueoussolution. Asweallknow,peoplehavevariedpreferences,particularly whenitcomestoclothing,wheretheyprefervariouscolors. Dyeisemployedinordertoproducevariouscolors.Methyl orange,Congored,malachitegreen,crystalblue,andother colorsare among the ones used. Fabrics are given specific colorsbydyeswhentheyareappliedtothem.
1.2 Release of dyes – Environmental impacts
Aftertheapplicationofdyes,theyarelosttoeffluentsand releasedintothewaterbodies.Thesedyesarenotremoved evenafterprimaryandsecondarytreatment.Inthetextile industry,differenttextilematerialscanbedyedusingbatch, continuous,andsemi-continuousprocesses.Theserequire different dyes, yarns, and fibres. Even though these dyes escapeconventionalwatertreatmentandarereleasedinto theenvironment.Becausedyescontaincolour,theywilltint thewaterwhentheyareintroducedintowaterbodies.Due to the properties of dye, they resist the biological degradation.Thedyesseriouslyaffectthetransparencyof water bodies and also their quality. This will eventually damagetheaquaticlifealso.
Some dyes decrease the light penetration, which decreases the amount of oxygen in the water. Apart from naturaldyes,azodyesaremostlyusedintextileindustries. These azo dyes can have toxic effects on the water and increasethelevelsofbiochemicaloxygendemand(BOD)and chemical oxygen demand (COD). If these dyes enter the human body, they will cause intestinal problems, liver problems, and many other harmful effects. Water that containsdyeshouldbecleansedbeforebeingreleasedintoa waterbody.Theimpactofthetextileindustryonwatercan bedevastating,butthegoodnewsisthattherearewaysto reduceitsdamagetotheenvironment.Physicalandchemical techniques, biological techniques, and sophisticated oxidationtechniquessuchasflocculation,precipitation,and
membraneseparationtechnologiesarecurrentlythemain ways to manage dye effluent. Adsorption among these systemshasdistinctbenefits,suchasabsorbingtheentire dyemoleculeandleavingnofragmentsintheeffluent,andis alsoacheapmethodfortheremovalofdye.

2 MATERIALS AND METHODOLOGY
2.1 Materials
Congo red (C3H22N6Na2O6S2), methyl orange (MO), ferric chloride(Fecl3),Ricestraw,Foodwaste,i.e.,vegetablepeels andfruitpeels,Distilledwater,Sodiumhydroxide(NaOH).
2.2 Methodology
2.3 Methods used
2.3.1 Pyrolysis

Pyrolysisistheheatingofanorganicsubstancelikebiomass withoutoxygen.Typically,biomasspyrolysisiscarriedoutat orabove500°C,whichprovidesenoughheattobreakdown thepreviouslystatedrobustbiopolymers.Becausethereis no oxygen, combustion cannot take place; instead, the biomassthermallybreaksdownintocombustiblegasesand biochar.
2.3.2 Adsorption
The phenomenon of attracting and holding onto a substance'smoleculesonthesurfaceofaliquidorasolid, resultinginalargerconcentrationofthemoleculesonthe surface, is known as adsorption. When charcoal is dipped intoacoloredsolution,thecolorofthesolutionfadesasa result of the charcoal adsorbing the colored particles. Adsorptionistheprocessbywhichthesurfaceofanother substance accumulates molecular, atomic, and discoloring ionicspeciesofafirstsubstance

2.4 Preparation of Food waste derived biochar


Collectdifferenttypesoffoodwaste.Thefoodwastecanbe anyvegetableorfruitpeel.Letthefoodwastedryintheoven at102degreesCelsiusfor24to48hours.Afterdrying,the food waste loses its moisture content. Now, cut the food wasteintofinepiecesandkeepitinamufflefurnaceusing cruciblesat500°Celsiusfor1hour.Nowthefinelycutpiece turnsintopowderandformsbiochar.



Take100mlofdistilledwater,add5gofbiochar,and0.5g ofFeCl3,andstircontinuouslyat130RPMfor2hoursusinga magneticstirrer.Duringstirring,add1moleofNaOHdrop wisetillthePHreaches9units.Filterthesolutionthrougha filter paper to separate the Fe-Biochar composite. Wash it with distilled water five times, which gives the Fe-Biochar composite. To get the biochar in a dry state, the biochar obtainedisovendried,whichgivesthebiocharinpowdered form.

2.5 Preparation of Fe-Biochar composite
Take 10g of rice straw and chop it into small pieces. Now take5%of10gofferricchlorideandmixitthoroughlywith the rice straw. Keep it in the muffle furnace for 30 min at 500°Celsius.Nowgrinditintofinepowder
2.6 Characterization of Food waste derived biochar and
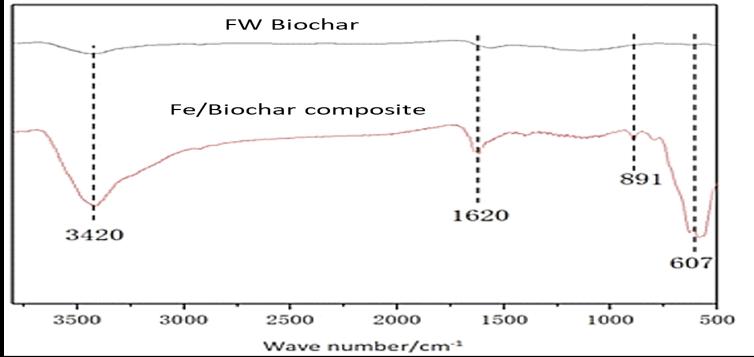
composite
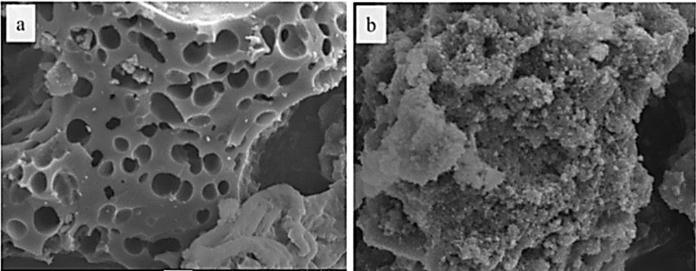
Surface morphology information of Food waste derived biocharandFe-biocharcompositewasobtainedbyscanning electronmicroscope
2.7 Removal of dye
2.7.1 Methyl orange
Take7.5mlofmethylorange.Addupto100mlofdistilled watertothemethylorangesolutiontodiluteit.Febiochar shouldbeweighedoutandthenaddedtothemixture.The same is true for biochar made from food waste. Stir the solutionconstantlyforthreehoursusingamagneticstirrer. Afterthat,filterthesolutionusingfilterpaperandtrackthe changes.Verifythesolution'sPHbothbeforeandafterthe experiment.Testothersamplesbyincreasingtheamountof Febiocharorthetimespentonamagneticstirreruntilthe dyeiscompletelyremoved,orperhapsupto70%ofit.The sameholdstrueforbiocharmadefromfoodwaste.
FT-IR information of Food waste derived biochar and Febiochar composite was acquired using the FT-IR electro



2.7.2 Congo red
Take1gofCongoredandadditto1000mlofdistilledwater. Now take 10 ml of the above solution and dilute it to 100ml.Weighconsecutivequantitiesofbiocharandadditto thesolution.Theprocessissameasthatofmethylorange theonlydifferenceismethylorangeistakeninliquidform while Congo red is taken in powdered form. Here in this projectazoanionicdyesaretested.
3. RESULTS
The total adsorption amount was calculated using the equationbelow:
qe (mg/g)= CO–Ce xV m
WhereCO andCe areinitialconcentrationsandequilibrium concentrationsofCRandMO(mgL-1),respectively;qeisthe equilibriumadsorptionamountofadsorbent(mgg-1);mand V are adsorption mass (g) and solution volume (L), respectively.
Removalrate(%)= CO–Ce X100 CO
Letusconsiderasample,foradurationof3hourswithan initialconcentration(C0)of30mg/Landusingtheadsorbent dose of 1 g/L, the maximum adsorption was calculated as follows:
CO =30mg/L
Ce =6.5mg/L
V=0.5L
m=0.5g
qe (mg/g)=(30–6.5)0.5 0.5 = 23.5mg/L
Removal(%)=78.3%
Thebelow-mentionedgraphistheresultoftheusageof Fe-biocharcompositeandFoodwastederivedbiocharin theadsorptionprocessformethylorangedye.
ThebelowmentionedgraphistheresultoftheusageofFebiochar composite and Food waste derived biochar in the adsorptionprocessforCongoreddye.
Food waste derived biocahr
Fe-biochar composite
GRAPH 4: RemovalrateofCongoredusingFe-biochar compositeandFoodwastederivedbiochar
4. CONCLUSION
Comparing the 2 bio chars used above, Fe-biochar composites have a higher removal rate than food wastederivedbiochar.Theadsorptionprocessusediseffectivein removalofazoanionicdyeresultinginanaverageremoval rateof75%.
5. REFERENCES
[1] Muhammad Bilal Shakoor, Shafaqat Ali, Muhammad Rizwan, Forahat Abbas, Irsad Bibi, Muhammad Riaz, Usman Khalil, Nabeel khan Niagi, Jorg Rinklebe; A review of biochar-based sorbents for separation of heavymetalsfromwater.
[2] Donlia Huang, Linshan Liu, Guangming Zeng, Piao Xu, Chao Huang, Linjing Deng, Rongzhong Wang Jia Wan; Theeffectofricestrawbiocharonindigenousmicrobial community and enzymes activity in heavy metals contaminatedsediments.

[3] Xianping Li, Chuanbin Wang, Jianguang Zhang, Juping Liu,BinLiuGuanyichen; Preparationofapplicationof magneticbiocharinwatertreatment-acriticalreview.
Food derived biochar
Fe-biochar composite
GRAPH 3: RemovalrateofMethylorangeusingFebiocharcompositeandFoodwastederivedbiochar
[4] JorgePazferreria,AnaMendes;Biocharfrombiosoilds pyrolysis.
[5] Vinoth Kumar Ponnuswamy, Gopala Krishnan Kumar; Review on sustainable production of biochar through hydro-thermalliquefaction:Physicochemicalproperties andapplications.
[6] Liang Zhanga, Iang Zhanga, YueLic; Preparation of an antibacterial chitosan coated biochar-nanosilver compositefordrinkingwater.
[7] Wenyang Ma, Wenyan Du, Zhubing Yan; Removal of PhloridzinbyChitosan-ModifiedBiocharpreparedfrom applebranches.
[8] FengiLiu,ShanHua,ChaoWang,MuqingQiu,LiminJiu, Baowei Hu; Adsorption and reduction of Cr (VI) from aqueous solution using cost effective caffeic acid functionalizedcornstarch.
[9] Nan Gao, Wanzhea Du, Manefese Zhang, Guixia Ling, Peng Zhang; Chitosan modified biochar preparation modifications,mechanisms&applications.
[10] Liwang, Yu jiao Wang, Fang Ma, Vistus Tan kpr, ShanshanBai,XiaoumengGuo,Xinwang;Mechanismand reutilization of modified biochar used for removal of heavymetalsfromwastewater.

