Hydrogen Fuel Cell AutoMobile: A Comprehensive Overview
 Suraj Kantheti1
S. Sai Koushik Goud2, M.K.Sai Srikar3
Suraj Kantheti1
S. Sai Koushik Goud2, M.K.Sai Srikar3
,
1 Student of Mechanical Engineering department, B V Raju Institute of Technology, Telangana, India

2 Student of Mechanical Engineering department, CMR Engineering College, Telangana, India
3 Student of Electronics and Communication Engineering department, Institute of Aeronautical Engineering, Telangana, India ***
Abstract - It is currently being thought about finding alternatives for obtaining energy by using technologies that give maximum efficiency and little pollution by harnessing the potential of renewable energy around the globe. New energy generation methods are required in this situation to both produce minimal carbon emissions and to fully utilize renewable energy sources. One of the other possibilities for upcoming sustainable energy systems is hydrogen fuel cell technology. This essay offers a thorough analysis of automobiles fueled by hydrogen fuel cells. It briefly explains the fuel cell theory, how current is generated in a fuel cell, the components of a fuel cell electric car, and overview of Hydrogen. The future scope of FCV are also covered, as well as the main advantages of FCV’s are also discussed, along with the principal challenges to ACV adoption and Further challenges are also covered. The relationship between the fundamentals and applications of fuel cells has been examined using data from both industry and academics.
Key Words: Hydrogen fuel cells, Emission, Ecofriendly, Fuel cell, hydrogen refueling, Electrolyzer, Proton exchange membrane fuel cell (PEMFC), Fuel cell Electric vehicle(FCEV)
1. INTRODUCTION
A hydrogen-containing fuel (water) reacts electrochemically with oxygen or another oxidizing agent to produce electricity in a fuel cell, which is an electrochemical device. Battery storage is used for producedenergy.afterwhichitisputtouseoutsideofthe company. They differ from batteries in that they need an ongoing supply of fuel and oxygen, typically from the air, to maintain the chemical reaction, whereas in a battery, the chemical energy comes from chemicals already existing in the cell. As long as fuel and oxygen are available, FCs can continually and uninterruptedly produce energy. In remote or difficult-to-access locations, theyprovidebackuppowerforindustrial,commercial,and residentialbuildings.
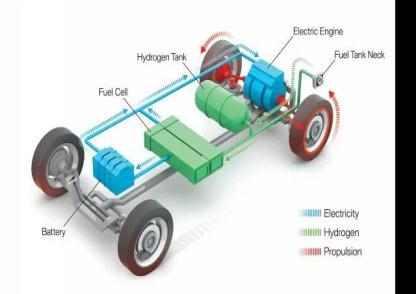
2. Working of a hydrogen fuel cell vehicles
Fuel cell electricvehicles(FCEVs) useenergyto power an electric motor, much like fully electric cars do. Unlike
other electric cars, FCEVs create their own electricity through the use of a fuel cell fuelled by hydrogen rather than relying only on a battery. The size of the electric motor(s) that get electric power from the suitably sized fuelcellandbatterycombinationestablishesthepowerof the vehicle during the vehicle design phase. Although automakers could design an FCEV with plug-in functionality to charge the battery, the majority of FCEVs today use the battery for energy recovery from braking, extra power during brief acceleration events, and to smoothoutthepowerdeliveredfromthefuelcellwiththe option to idle or turn off the fuel cell during low power needs.Thesizeofthehydrogenfueltankaffectshowmuch energy can be stored on board. An all-electric car, on the otherhand,hasastrongrelationshipbetweentheamount of power and energy available and the size of the battery.[1]
The chemical energy that hydrogen fuel stores is converted into electricity by a hydrogen fuel cell. A fuel cellwithasupplyofhydrogenandoxygenmaybeusedto power electrically powered equipment, much like a battery. Fuel cells and batteries both transform chemical energy into electrical energy, but batteries store this chemicalenergywithinthebatteryitself.Thisimpliesthat a battery will become exhausted or need to be recharged when there is insufficient chemical energy in storage to provide enough electricity to operate the connected device. A hydrogen fuel cell gets its source of chemical energy fromtheoutside ratherthanstoring it within.The
hydrogen that is given to the fuel cell's anode stores this chemical energy. In essence, hydrogen and oxygen are used by a hydrogen fuel cell. A fuel cell may produce electricityifit receivesconstantsuppliesofhydrogenand oxygen and is not exposed to water throughout the production process. Batteries and hydrogen fuel cells are bothtypesofelectrochemicalcells.Theyeachcontaintwo electrodes in contact with an electrolyte, which is a substance that can conduct ions. The anode is one electrode, while the cathode is the other. In contrast to batteries, which release electrons from the substance inside the anode, hydrogen fuel cells release electrons from the hydrogen that is delivered to the anode. Battery electrodes actively contribute to the conversion of chemical energy into electrical energy, which over time may have a negative impact on the electrodes and, consequently,thebattery'sefficiency.Purewaterandheat are the sole byproducts of the fuel cell when pure hydrogenisemployedasthefuel.Sincetheyhavenoeffect on the environment, fuel cells have the potential to be incredibly efficient devices. Both of these by-products are frequentlyusefulinsomeway.Theheat,forinstance,may be applied wherever heat is required. Since the Gemini programme in the 1960s, fuel cells have been utilized in NASA spacecraft, and they are still used today to power and hydrate personnel on Space Shuttle missions. Since hydrogen is not naturally present in the environment, hydrogen fuel must be produced from substances like methanol, gasoline, natural gas, and water that contain hydrogen. Today, natural gas is used to manufacture the majority of the hydrogen. The only result of producing hydrogen from water is clear water. There will be more byproducts, such carbon dioxide, if fossil fuels are the hydrogen'sprimarysource.Electricityisusedtodividethe water molecule in order to make hydrogen from water. The generated hydrogen is a renewable, zero-emission fuel if the electricity originates from a renewable energy source, such wind or solar power. Although it may be given in pure form, oxygen isgenerallytaken through the air.[3]
4. Working of hydrogen fuel cell

The anode of the fuel cell receives hydrogen gas. The anode is plated with platinum, which functions as a catalyst to break down hydrogen into protons and electrons. If a circuit is linked between the anode and cathode,electronscanmoveacrossthecircuitanddeliver powertoanyloadthatisconnectedaspartofthecircuit. Theanode’sresponse:
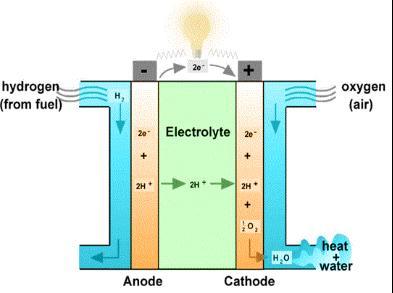
2H2→4H++4e−
Theelectriccurrentgeneratedbythefuelcellistheflowof electrons through the load. The hydrogen ions (protons) generated from the hydrogen at the anode go from there to the cathode through the electrolyte of the fuel cell. These hydrogen ions and electrons from the external circuit combine with the oxygen delivered to the cathode tocreate waterandheat, bothof whichare expelled from thefuelcell.
Thecathode'sresponse:
O2+4H++4e−→2H2O
Bipolar plates are positioned on the opposite side of the cell in a PEM fuel cell. They act as current collectors and aid in gas distribution. Between the anode and cathode, which are all sandwiched between the bipolar plates, is a membrane that houses the electrolyte. Proton exchange membranes,orPEMs,aremembranesthatonlyletprotons flowthroughthem.Themembranehasto be wet inorder tofunctioncorrectly.
The normal output of a hydrogen fuel cell is 0.5 to 0.8 volts per cell. Individual cells may be linked in series to raise the voltage. The structure in question is known as a fuel cell stack. The capacity of a fuel cell to generate currentdependsonitscrosssectionalarea.Moreresponse sites may be created since a larger region has more of them. Power equals current times voltage. Therefore, it is feasibletocreateveryhugeamountsofelectricalpower enough to power an entire neighborhood of homes, a hospital,oravehiclelikeacar,bus,orevenasubmarineor spacecraft by stacking cells in series to increase voltage andincreasingcellareatoenhancecurrent.[3]
5.Key Components of a Hydrogen Fuel Cell Electric Car
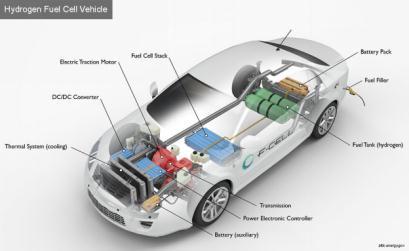
oxygen, electric motors are introduced. The car moves along with minimum noise and vibration thanks to the electric motor. It can also gain energy by slowing down. The fuel cell and battery output and input are controlled by the power control unit depending on the driving situation.
Thefuelcell'soutputofpowerDependingonthedemands of the particular driving situation, a hydrogen engine can travelinoneoftwodirections.Iteithergoestotheelectric motor and immediately drives the FCEV or it charges a battery that saves the energy until the engine needs it. Because the fuel cell frequently recharges this battery, which is referred to as a peak power battery, it is substantially smaller and lighter than the battery of a completelyelectricvehicle.[5]
Fig-3: KeycomponentsofHFCV
Battery pack: In addition to supplying additional power to the electric traction motor, this high-voltage battery storesenergyproducedduringregenerativebraking.
DC/DC converter: The traction battery pack's highervoltage DC power is converted by this device into the lower-voltage DC power required to operate the vehicle's accessoriesandreplenishtheauxiliarybattery.
Electric traction motor (FCEV): This motor moves the wheelsofthevehiclebydrawingenergyfromthetraction battery pack and fuel cell. Some automobiles employ motor generators that serve as both drives and regenerators.

Fuel cell stack: An arrangement of individual membrane electrodes that generate electricity from hydrogen and oxygen.
Fuel filler: Tofillthetank,agasolinedispenser'snozzleis connectedtothevehicle'sreceptacle.
Power electronics controller (FCEV): This device controlstheelectrictractionmotor'sspeedand torque by managing the flow of electrical energy produced by the fuelcellandthetractionbattery.
Thermal system (cooling) - (FCEV): Thefuelcell,electric motor, power electronics, and other parts of the system are all kept within a safe operating temperature range by thissystem.
Transmission (electric): To move the wheels, the transmission converts electrical traction motor output intomechanicalpower.[1]
5.1 Electric motor and battery
In hydrogen fuel cell automobiles, where the power is produced by the chemical reaction of hydrogen and

5.2Hydrogen fuel tank
The hydrogen storage tank is the component of the HFCV that is the most dependable, secure, manageable, and economical. In comparison to other fuels, hydrogen has a low energy density that is sustainably lower. Its energy per volume is significantly lower than that of liquid fuels like gasoline. In the past, an HFCV could travel 300 miles on about 5 kg of hydrogen, but today, this requires a fuel tank that is 3–4 times larger than that of gasoline. The main challenge is figuring out how to store the hydrogen.Todevelopandvalidatetheonboardautomotive hydrogen system, which must meet consumer demands and expectations for range, passenger and cargo space, refueling time, and overall vehicle performance, several types of research and development are being conducted using fuel cell technology. Toyota has developed a tweak thatallowsfor1.7timesasmuchhydrogentobestoredin 350bartank
As opposed to 700 bar tanks, giving vehicles a driving rangeofmorethan310miles.[5]
6. FCV Emissions
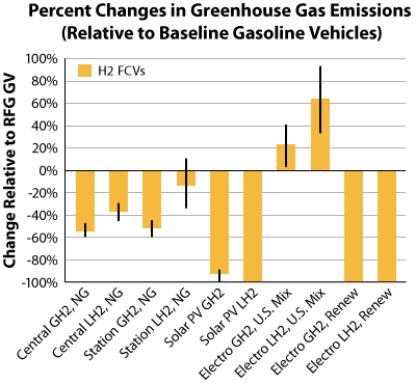
The only pollutants produced by fuel cell electric cars are water vapor and warm air, with no tailpipe emissions. Hydrogen,likeelectricity,isanenergycarrierthatmaybe created from a variety of feedstocks. When calculating hydrogen emissions, these feedstocks and manufacturing processesmustbetakenintoaccount.[1]

pressure tanks capable of holding hydrogen at 5,000 or 10,000 pounds per square inch (psi). For example, the FCEVs now in production and available at dealerships have 10,000 psi tanks. Retail dispensers, which are often foundnearpetrolstations,canfillthesetanksinaround5 minutes. Currently, fuel cell electric buses have 5,000 psi tanksthattake10-15minutestofill.[1]
7. Overview of Hydrogen
Hydrogen,whenusedinafuelcelltogenerateelectricity, is a zero-emission alternative fuel derived from a variety of energy sources. Drivers of light-duty fuel cell electric cars(FCEVs)canalreadyfillupinlessthan5minutesand have a driving range of more than 300 miles. There are currentlyresearchandcommercialactivitiesunderway to extend the limited hydrogen fuelling infrastructure and increaseFCEVmanufacturing.
The Energy Policy Act of 1992 defines hydrogen as an alternativefuel.Theabilityofhydrogentopowerfuelcells in zero-emission cars, the possibility for domestic production, and the fuel cell's rapid filling time and high efficiency all contribute to the interest in hydrogen as an alternative transportation fuel. In fact, a fuel cell paired withanelectricmotoristwotothreetimesmoreefficient than a gasoline-powered internal combustion engine. Hydrogenmayalsobeusedtopowerinternalcombustion engines.These,however,createexhaustemissionsandare less efficient than FCEVs. Find out more about fuel cells. The energy content of 2.2 pounds (1 kilogramme) of hydrogen gasisaboutequivalentto theenergy content of 1 gallon (6.2 pounds, 2.8 kilogrammes) of gasoline. Because hydrogen has a low volumetric energy density, it isstoredasacompressedgasonboardavehicletoprovide the driving range of conventional automobiles. The majority of modern applications make use of high-
8. Types of hydrogen
Thethreemostcommontypesofhydrogenaregray,blue, andgreenhydrogen.
Gray: Grey hydrogen generation is now the most popular and least expensive method. Although it doesn't produce greenhouse gas emissions on its own, the process of makingitdoes.Itisusedasafuel.Steamreforming,which separates the hydrogen from the natural gas, is used to produce gray hydrogen from natural gas. The carbon emissions produced during the process are not, however, captured by the technologies utilized and are instead dischargedintotheatmosphere.
Blue: Thesteamreformingmethodisused toextractblue hydrogen, but it varies from gray hydrogen in that the emittedcarbonemissionsarecaughtandstored,reducing but not completely eliminating the emissions in the atmosphere.Duetothefactthattheproductiontechnique juststoresgreenhousegassesratherthanpreventingtheir generation, blue hydrogen is frequently referred to as "low-carbonhydrogen."
Green: Green hydrogen is a real source of clean energy since it is produced from renewable resources, which resultsinzeroemissionsthroughoutitsentirelifecycle.It is produced by electrolyzing water with clean electricity produced from extra sustainable wind and solar energy. The procedure results in a reaction that separates water intoitshydrogenandoxygencomponents(theHand Oin H2O). As a consequence, there are no carbon emissions produced during the process. Although it's a fantastic replacementforgrayandblue,thekeyobstaclerightnow
is lowering the price of green hydrogen generation in order to make it a really affordable renewable energy source.
Black and Brown: Usinganykindofcoalintheextraction process results in the production of black and brown hydrogen.Theelectrolysisofgreenhydrogenisoneendof a spectrum, while this process, called gasification, is the other. It is a well-known method that is employed in several sectors to transform materials rich in carbon into hydrogen and carbon dioxide. The emissions are subsequentlydischargedintotheatmosphere,wherethey cause pollution and turn into the most environmentally hazardousformofhydrogen.
Pink: Nuclear energy is used for the electrolysis process that extracts pink hydrogen. Pink hydrogen is sometimes referredtoaspurplehydrogenorredhydrogen.
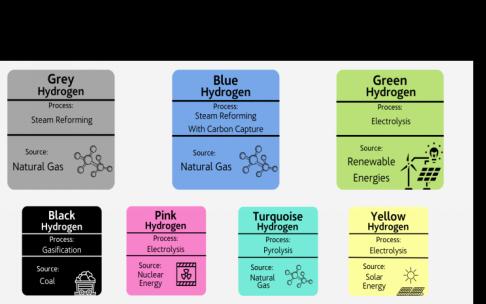
Turquoise: Turquoise hydrogen is still being researched toseeifitcanbeeffectivelyemployedonabroadbasis.It isproducedbyaprocedureknownas"methanepyrolysis," which uses heat to decompose a substance's chemical structure to yield hydrogen and solid carbon. Instead of being released into the atmosphere, carbon is kept in the solid carbon that is produced. If turquoise is shown to be successfulandthecarboncanbepermanentlystoredinan environmentally safe manner, it may join blue as a "lowcarbonhydrogen."
Yellow: Yellow hydrogen is produced by electrolysis particularly using solar energy, much to the method used toproducegreenhydrogenbutwithacheeriermoniker.
White: Undergroundgeologicalhydrogendepositscontain naturally occurring white hydrogen. By drilling into the ground and injecting a highly pressurized solution of water, sand, and chemicals at the rock to liberate the gas inside, the procedure is known as fracking. There are no planstoutilizethiskindofhydrogenasasourceofenergy atthetime.[2]
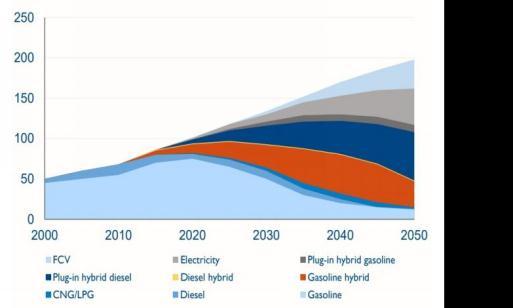
9. Future scope of FCV
Despite a positive outlook for climate legislation, considerable FCV sales volumes are only anticipated over the long run. Following the approval of the Paris Agreement, hopes for the future FCV market are increasing due to a recognised lack of CO2 emissions duringvehicleoperation.
Graph-3: FCVsalespredictions
Source: International Energy Agency 2012 (IEA) According to a comparable scenario, the international energy agency (IEA) forecasts a 35 million unit annual salesFCVmarketshareofroughly17%by2050.[4]

10. Principal challenges to FCV adoption
Three primary issues, including the cost of cars, distribution infrastructure, and hydrogen generation, are impedingthepotentialadoptionofFCVs.
Cost of vehicles: The usage of costly catalysts and other materials in the fuel cell "stack" is the major cause of the highcostofFCVs(e.g.platinum).
Distribution infrastructure: Important and costly investment choices are necessary for the growth of infrastructure, and they must be backed by ongoing FCV marketdemand.
Hydrogen production: Similar to how electricity is produced, hydrogen may be produced using a variety of basicenergysources, each ofwhichhasa varied effect on theGHGfootprint.
The hydrogen can be classified as "green" or "gray" depending on the main energy source from which the energy is generated. Producing hydrogen from renewable primary energy sources (often referred to as "green") as opposed to "gray" resources is more advantageous in termsofGHGimpact.[4]
11. Main advantages of FCVs
FCVspromiseadvantagesacross a rangeofparametersin addition to the absence of CO2 emissions during vehicle operation:
Refueling time: Tofillthetank,itwilltakeafewminutes (as for ICE engines), as opposed to the lengthier time anticipatedforbattery-electriccarstorecharge(BEVs).
Driving range: Withadrivingrangeofmorethan450km, FCVs are already competitive on the market and, on average,havelongerrangesthanBEVs.
Fuel efficiency: FCVs utilize roughly 40 to 60 percent of the energy included in hydrogen, compared to about 20 percent in ICE cars, making them more energy efficient than gasoline-powered vehicles. It's vital to note that EVs use around 75% of the energy from the batteries, which makesthemmoreefficientthanFCVs.
Scalability: It is simple to increase the power of FCVs: to create a fuel cell stack, which can generate enough electricity to power a vehicle, separate fuel cells are connected in series. This feature of the technology makes itpossibletoemployitinheavy-dutyvehiclesaswell.
Weight and volume of energy storage: A lithium-ion batterysystemrequiresaroundsixtimesmoreweightand twice the space to permit comparable driving ranges (for example, 500 km), whereas H2 requires less weight and volume for energy storage to provide the same distance range.
Sustainability: In addition to producing no greenhouse gasses while the vehicle is in motion, FCV drive batteries are smaller than those of BEVs, resulting in a reduced environmental effect from the use of heavy metals in the production of Li-ion battery packs. However, pollution from power plants should be contrasted with pollution from the H2 generation process (which depends on the primary energysourceusedtocreateit)whencomparing FCVswithBEVs.[4]
12. Further challenges
Durability and reliability: It will be necessary for FCV livestobeequivalenttothoseoftraditionalpassengercars (e.g.approximately14years)
Safety and public acceptance: Thepressurizedstorageof hydrogen on-board vehicles is one area of concern. H2 is invisible to the human eye, nose, and tongue since it has notaste,smell,orcolor.
Onboard hydrogen storage: InordertostoreenoughH2 tocreatealong-rangevehicle,averybigtankorextremely highlypressuredtanksarerequired.[4]
13. CONCLUSIONS
Since the only byproducts of hydrogen fuel gas powered carsarewaterandheat,theyareanexcellentsolutionfor reducingpollutionandgreenhousegasemissions.Because hydrogen has a higher energy storage capacity, it takes less time to recharge the car and has a longer driving range. The only challenge is obtaining hydrogen. The conventional technologies for obtaining hydrogen have thedrawbacksofsignificantenergyloss,limitedefficiency, and environmental degradation. However, other techniquesofcreatinghydrogenarebeingdeveloped,such as the proton exchange membrane, which experts believe has an 86% efficiency. Using the extra energy source for hydrogen production and developing a hybrid version of hydrogen-lithium-ion automobiles may potentially be something
REFERENCES
[1]https://afdc.energy.gov/vehicles/fuel_cell.html
[2]https://www.acciona.com.au/updates/stories/whatare-the-colours-of-hydrogen-and-what-do-they-mean/
[3]https://sepuplhs.org/high/hydrogen/hydrogen.html
[4]ArthurD.Littlefutureoffuelcellvehicles. https://www.adlittle.com/sites/default/files/viewpoints/ ADL_Future%20of%20Fuel%20cell%20vehicles.pdf
[5]KancharaPadmaRao,PadalaTejaswi.2020.“Hydrogen Fuel Cell System Automobiles:A Mode to the Recent Scenario”, International Research Journal of Engineering and Technology[IRJET]. Volume: 07, Issue: 09. e-ISSN: 2395-0056,p-ISSN:2395-0072
[6] Cissy Shaji, Deepa P, Samir Nimkar. 2021. “Hydrogen Fuel Cell Powered Vehicles”, International Research Journal of Engineering and Technology[IRJET]. Volume: 08,Issue:05.e-ISSN:2395-0056,p-ISSN:2395-0072
[7]YasuhiroNonobe.2017. “Developmentof the Fuel Cell Vehicle Mirai”. Wiley Online Library. DOI: 10.1002/tee.22328

