International Research Journal of Engineering and Technology
(IRJET) e-ISSN: 2395-0056

Volume: 10 Issue: 1 | Jan 2023 www.irjet.net p-ISSN: 2395-0072
ANALYSIS OF HYDROGEOCHEMICAL AND MINERALOGICAL CHARACTERISTICS RELATED TO HEAVY METAL ATTENUATION IN A STREAM POLLUTED BY ACID MINE DRAINAGE: A CASE STUDY
D. Sharma1 , Dr. A. Bhatnagar2
1M. Tech. Student, Dept. of Mining Engineering, CTAE, MPUAT, Udaipur, Rajasthan, India.
2Professor and Head, Dept. of Mining Engineering, CTAE, MPUAT, Udaipur, Rajasthan, India. ***
Abstract - Acid mine drainage discharged from the mine may be harmful for the environment as well as for the human beings. The study area comprises of underground metal mine working and the Banas river which flows nearby the mining area. In this study, chemical characteristics if the water samples, mineralogy of the AMD sediments and heavy metal attenuation are discussed on the basis of mineral analysis, chemical analysis and sequential extraction techniques. The chemical characteristics of the acid mine drainage andsulfide minerals from the mine tailings are determined in order to study the impact of acid mine drainage of the river water. The water samples are classified as affected and unaffectedwater. The affected water has low pH value, high (SO4)2- ions and high heavy metal concentration. The concentration of heavy metals and sulfide ions decreases and the pH increases downstream. The acid mine drainage water chemistry is controlled by the transformations and mineral precipitates of Fe3+ minerals.
Key Words: Acid mine drainage, Sediments, Mineralogy, Attenuation, Sequential extraction, Heavy metal
1.INTRODUCTION
Mininghasbeenimportantpartinhumanworld.Itprovides variousmineralsandcoalthathasbeenusedinregularbasis e.g.ThermalPowerPlants,CementIndustryetc.Butthere existsvariousproblemslikeAcidMineDrainage.Itisoneof themajorproblemspollutingthewaterasitpollutesaround 27billionofwaterperyear.Humanhealthhasbeenmajor issuewhichsurroundstheminearea.
Aminedrainingacidcandestroystreams,rivers,andaquatic lifeforhundredsofyears.Oxidationandliberationofsulfur present in rocks in the form of sufide minerals generates sulfuric acid. This is one of the major impacts of coal and metal mining activity. Acid Mine Drainage (AMD) is a problemthatinitiateswithinshorttimeinsulfiderichmines. Whenthesesulfidemineralscomesincontactwithoxygen oroxygenatedwaters,breakdownofthesemineralsleadsto acidgenerationandleachingofmetals.
Acid mine drainage leads to the problems like contaminated drinking water, disrupted growth and reproduction of aquatic plants and animals and the
corroding effects of the acid on infrastructures such as bridges, etc. Treatment of AMD by conventional methods include various physicochemical methods, which involve excessiveuseofchemicalsandcapital.Biologicaltreatment has come out as efficient, cost-effective, and eco-friendly alternative for remediation of AMD. Biological treatment methodsinvolveuseofmicroorganismssuchasbacteriaand fungi. Biotechnological approaches can prove an asset in developingtechniquesthatcantreatAMDinaneffectiveway withoutaffectingtheenvironmentalsustainability.
Heavymetal contaminationhasslowlybecomea common and important concern world-wide. The most common heavy metals are iron, copper, zinc, lead, nickel, arsenic, mercuryandcadmium.Heavymetalsaredistributedinthe aquatic environment as sedimentary phases, suspended forms,colloidsandwater-solublespecies.Heavymetalsare an important category of pollutants as they can have a significant harmful effects on both human health and the health of terrestrial and aquatic communities and ecosystems.Anumberofheavymetalshavebeencommonly studied as pollutants due to high environmental concern, suchasCd,Cu,Cr,Hg,Pb,Ni,andZn.
Thedangerofheavymetalpollutioncanbeunderstandby threefundamentalreasons.Theyarenotbiodegradable,so they remain indefinitely in the environment, unless transportedtootherenvironments.Theycanberetainedby organic tissues through bioaccumulation and then transmittedtootherspeciesina higherlevel of thetropic chain,thuscausingbiomagnifications.Someofthemsuchas copper,zincandmanganesearenecessarymicronutrients for some plants and animals, but they can become lethal above certain concentration levels. However, organisms need these metals within an optimal level, under which conditionsofwantappearandoverwhichwefindtoxicity.
Themineinthestudyareahasbeenextensivelyminedwith massivetailingsleftbehindandthesurroundingshaveborne the effects of AMD contamination. Production of AMD continuesinthetailingreservoir.AMDdischargefromthe minehascausedsevereenvironmentalpollutionandhuman healthproblems.
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page80
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 10 Issue: 1 | Jan 2023 www.irjet.net p-ISSN: 2395-0072
2. METHODOLOGY
2.1 Field Work
Thirtyfivewatersampleswerecollectedacrosstheriverin theregionofminingarea.Acompositesamplewasformedat eachsamplingsitebycollectingthreesub-samples.Onthe basis of the geological conditions and hydrodynamic relationships,watersamplesareclassifiedintotwogroups: affectedwaterandunaffectedwater.
Ninesedimentsampleswerecollectedwhichincludetailing dam sediment, waste dump sediment and streambed sedimentsalongtheBanasriver.Thesurfacewatersampling siteswereco-locatedwiththesedimentsamplelocations.A composite sample was formed at each sampling site by collectingfivetoplayersedimentsamplesofthickness10cm withintheareaof5m2
2.2 Laboratory Work
Temperature, pH, Electrical conductivity (EC), oxidationreduction potential (ORP), total dissolved solids were measured in the laboratory were measured in-situ with portableinstrument(thermoorion4star).Theconcentration of the dissolved ions were measured using conventional methods. The concentration of heavy metals were determinedbyinductivelycoupledplasma–opticalemission spectrometry(ICP-OES).Mineralogywasdeterminedbyfield emissionscanningelectronmicroscopy(FE-SEM),powderxraydiffraction(XRD)andfouriertransforminfrared(FT-IR).
3. FIELD AND LABORATORY INVESTIGATIONS
3.1 Analysis of Dissolved Ion Concentrations and TDS
Conventional methods were used for determination of dissolvedionconcentrations.Alkalinitywasdeterminedby acid–basetitrationmethodwithHClsolution(0.025mol/L) at a pH of 4.5 – 4.6, within 24 hours after the sample collection. Triplicate analysis was performed to test the precision and accuracy of the computed results. TDS was measuredin-situwithportableinstrument(thermoorion4starmeter).
3.2 Determination of pH, Electrical Conductivity (EC) and ORP
pH, electrical conductivity (EC) and oxidation-reduction potential (ORP) were measured in-situ with the portable instrument,thermoorion4-starmeter.
3.3 Analysis of Heavy Metals and Trace Elements
Inductivelycoupledplasma–opticalemissionspectrometry (ICP–OES)wasusedtomeasuretheconcentrationsoftrace
elements and heavy metals. Other metals analysis proceduresarelistedintable1.
Table-1: Analysis methods of different metals
Metal Analysis Method
K,Na,CaandMg Atomicabsorptionspectrometry FeandFe2+ Colorimetry
As Atomicfluorescencespectrometry
3.4 Analysis of Mineralogy of AMD Sediments
Before the analysis, sediment samples were pulverized. Mineralogy was determined by field emission scanning electron microscopy (FE-SEM), powder X-ray diffraction (XRD)andfouriertransforminfrared(FT-IR).
3.5 Sequential Extraction Method
0.2 gm of dry sediments were taken and sequential extractions were performed. The details of the analysis procedureareshowninthetable2
Table-2: Sequential extraction procedure used in this study
Fraction Extraction (dilution) Procedure
Exchangeable fraction 1mol/LMgCl2 (pH=7) Continuous shaking for 2 hours (room temperature)
Adsorbed carbonates 1mol/L NaOAc(pH= 5)
Continuous shaking for 3 hours (room temperature)
Fe-Mnoxides 0.04mol/L NH2OH-HCL Heat in water bath 96ºCfor6hours
Organicmatter 0.04mol/L HNO3-H2O2 and3.2mol/L NH4OAc
Residual
30% H2O2 added twicetosampleswith 0.04 mol/L HNO3, shakinginwaterbath 85ºCfor3hours,cool toroomtemperature, thenadded3.2mol/L NH4OAc for 30 minutes
HCl-HNO3HClO4 Sampledigestedwith HCl, HNO3 and HClO4 in a microwave digester
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 10 Issue: 1 | Jan 2023 www.irjet.net p-ISSN: 2395-0072
4. RESULTS AND DISCUSSION
4.1 Chemical Characteristics of Water
The main characteristics of the affected water are high concentrationsof(SO4)2- andTDS
Table-3: Geochemical parameters of water smaples
Table-5: Correlation values of EC with different ions Ions Correlation with EC
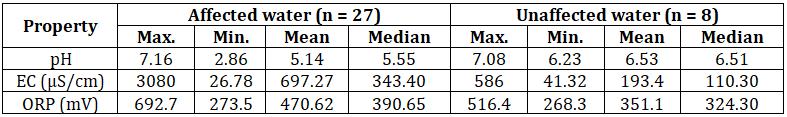
Ca2+ 0.85 Mg2+ 0.95 (SO4)2- 0.94 0 50 100 150 200 250 300 350 400 450 500
TheconcentrationsofHCO3-,(SO4)2-,(Cl)1-,Mg2+,Na+ andK+ variesinaffectedwaterandchangeduniformlywithdistance from the source of AMD. The concentration of Ca2+ was abundantinaffectedaswellasunaffectedwater.
The average concentration of HCO3- (44.12 mg/L) in the study area was significantly lower in affected water as comparedtounaffectedwater(71.42mg/L).Onthecontrary, theaverageconcentrationof(SO4)2- ionsismoreinaffected water.Thisshowsthatmore(SO4)2-isreceivedintheaffected waterfromtheminetailings.
4.2 pH, Electrical Conductivity (EC) and ORP
Generally,thechemicalprocessesofAMDismainlyinfluence by the pH. In unaffected water, the highest pH value measured was 7.08 while in affected water, the lowestpH measuredwas2.86.ThepHvalueinthestudyareaincreased downstream of the Banas river because unaffected water flowedintotheaffectedwater.
Table-4: pH, EC and ORP parameters of water samples
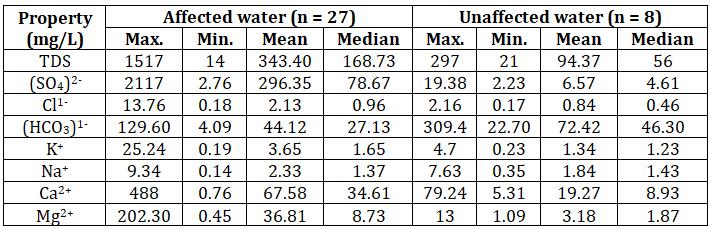
Concentration (mg/L) EC (µS/cm)
0 1000 2000 3000 4000
Chart-1: Correlation plots of EC vs Ca2+
ThehigherECvaluesinaffectedwatershowsthataffected water contain more minerals as compared to unaffected water.ECvalueshavegoodcorrelationswithCa2+,Mg2+ and (SO4)2- concentrations as shown in the chart 1, 2 and 3 respectively.
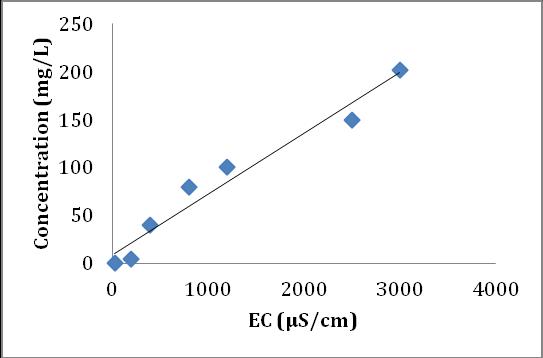
Chart-2: Correlation plots of EC vs Mg2+
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page82
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 10 Issue: 1 | Jan 2023 www.irjet.net p-ISSN: 2395-0072
Table-7: Correlation of Al with other elements
Concentration (mg/L)
2000
1500
1000
500
0
2500 0 1000 2000 3000 4000
EC (µS/cm)
Chart-3: Correlation plots of EC vs (SO4)2-
4.3 Heavy Metals and Trace Elements
As AMD is discharged at mining sites, sediment is an important factor. In understanding the environmental activities of trace elements and heavy metals in AMD, sedimentgeologyandmineralogyareveryhelpful.
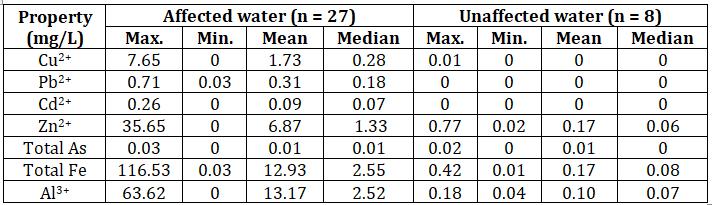
TheaffectedwatercontainshighconcentrationsofCuandZn withlowconcentrationsofCd,AsandPb.
Table-6: Heavy metal concentrations
Elements
Correlation (R2)
Al:Zn 0.98
Al:Cu 0.94
Al:Pb 0.97
Al:Cd 0.97
Al:Mn 0.85
4.4 Mineralogy of AMD Sediments
The sediments of Fe minerals were found at tailing dam, wastedumpsandriverintheminingarea.Thesedimentson riverbanks in AMD affected areas consist of Fe phases precipitated from Fe dissolved in AMD which comes from pyriteattheminesite.
The minerals of AMD sediments consist of amorphous hydroxides and oxyhydroxysulphates, such as as schwertmanniteandgoethite.AstheAMDleachesfromthe mine,secondarymineralsareformed.
Schwerrtmanniteisthedominantmineralatwastedumpsite andtailingdambecauseofthecontinousinjectionandslow movementofAMD.Goethiteandquartzwithlowamountof schwertmannitearepresentatriverbanks.Thisshowsthat themineralschangedfromschwertmannitetogoethiteand the amountofgoethite increases with increase indistance fromtheminetailings.
Thetransformationoccursviathefollowingreaction:
Fe8O8(OH)5.5(SO4)1.25 +2.5H2O=8FeOOH+2.5H+ + 1.25(SO4)2-
4.5 Sequential Extraction of Sediment
CadmiumexistsinsphalritealongwithZnandthegeological characteristicsofCdandZnaresimilar.Theconcentrations of Cd and Zn were well correlated in affected water (R2 = 0.97,n=27).
Thecorrelationsbetweenotherelementsand Alwerealso significantandareshowninthetable7.Thisindicatesthatat the mine site, these elements originated from the same minerals.
The exchangeable and organic matter fraction of Zn decreased and carbonate & Fe-Mn oxide increased downstreamintheriver.TheresidualphaseofZndominated intheBanasriversediments.Organicmatterphasedominate neartheminesitewhileFe-Mnoxidebecamethemainphase aswemovedawayfromthemine.
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page83
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 10 Issue: 1 | Jan 2023 www.irjet.net p-ISSN: 2395-0072
100%
80%
60%
40%
20%
0%
1 3 6 9 11 15 18
Chart-4:
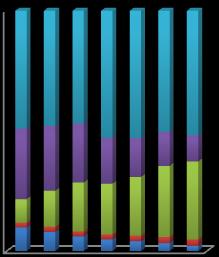
Residual Organic matter Fe-Mn Oxide Carbonate Exchangeable
100%
80%
60%
40%
20%
0%
Sequential extraction of Zn
The organic matter and carbonate phases increased while exchangeablefractionsdecreaseddownstreamoftheriver. Fe-MnphaseofCuincreasesupto9th samplinglocationand thendecreasesdownstreamoftheriver.Organicmatterwas themajorfractionforCuthroughoutthesamplinglocations.
100%
80%
60%
40%
20%
0%
1 3 6 9 11 15 18
Residual Organicmatter Fe-Mn Oxide Carbonate Exchangeable
1 3 6 9 11 15 18
Residual Organic matter Fe-Mn Oxide Carbonate Exchangeable
Chart-6: Sequential extraction of Cd
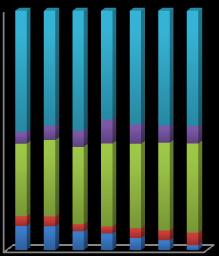
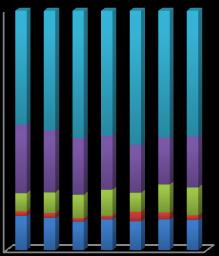
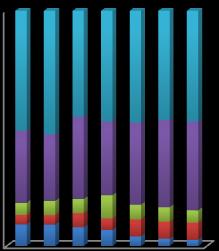
Chart-5: Sequential extraction of Cu
Cdwasmainlyfoundinresidualphase(silicate)andFeoxide phases. The organic and exchangeable fractions of Cd decreased while carbonate and Fe-Mn oxide fractions increasedaswemovedawayfromtheminesite.Thefraction ofresidualphaseforCdwashighestascomparedtothatof Zn,PbandCu.
80%
60%
40%
20%
100% 1 3 6 9 11 15 18
Residual Organic matter
Fe-Mn Oxide Carbonate Exchangeable
The organic matter fraction for Pb increases and then decreasesaswemoveawayfromtheminesite.Carbonate andFe-Mnoxidephasesincreasedwhileexchangeablephase decreased downstream in the river. Fe-Mn oxide is the dominantfractionforPb. 0%
Chart-7: Sequential extraction of Pb
4.6 Attenuation of Heavy Metals in AMD
Heavy metals in AMD can be removed by adsorption, deposition, co-precipitation and bioremediation. The adsorption,depositionandco-precipitationarephysiological processes and their removal efficiency of heavy metals is affectedbythepHvalues.
Fe or Al hydroxide minerals plays important role in the removalofheavymetalsandotherionsbyadsorptionand co-precipitationprocess.Fehydroxidessurfacecanadsorb Cu,ZnandPb.Alhydroxidesorhydroxysulphatescanadsorb Cu,Zn,PbandNi.
AMD can be neutralized by dissolution of carbonate minerals. Initially, AMD of the mine is strongly acidic
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 10 Issue: 1 | Jan 2023 www.irjet.net p-ISSN: 2395-0072
because sulphide oxidation results in the liberation of H+ ions. As the AMD moves away from the mine tailings, carbonate minerals buffer the acidity produced by sulfide minerals resulting in increase of the pH and decrease of concentrationsofheavymetalsinthewater.
Due to the above natural attenuation processes such as adsorptionandco-precipitationofsecondarymineralsand buffering by carbonate minerals, the hydrogeochemical characteristic of the affected water become similar to the unaffectedwateroftheBanasriver.
3. CONCLUSIONS
In the surrounding of study area, the chemical characteristicsoftheaffectedwaterarecontrolledbysulfide minerals rather than belonging to carbonate type. AMD is characterizedbylowpHandhighconcentrationsofheavy metalsandhighconcentrationsofSO42-.Ca2+ andSO42-were the major ions present in the affected water while concentrationsofCa2+ andHCO3- dominatedintheaffected water.
The concentrations of heavy metals and SO42- decreased downstreamintheriverwhilepHincreases.Theresultsof XRD, FT-IR, FE-SEM and and sequential extraction experiment revealed that secondary Fe minerals were precipitatedintheaffectedwater.Atthewastedumpsite, schwertmannitewasthemajormineralswhilegoethitewas themajormineralatthetailingsdamandriverbanks.
The exchangeable and organic matter fraction of Zn decreased and carbonate & Fe-Mn oxide increased downstreamintheriver.Theorganicmatterandcarbonate phasesCuincreasedwhileexchangeablefractionsdecreased downstreamof the river. Cd wasmainlyfound in residual phase (silicate) and Fe oxide phases. The organic and exchangeablefractionsofCddecreasedwhilecarbonateand Fe-Mnoxidefractionsincreasedaswemovedawayfromthe minesite.TheorganicmatterfractionforPbincreasesand then decreases as we move away from the mine site. Carbonate and Fe-Mn oxide phases increased while exchangeablephasedecreaseddownstreamintheriver.FeMnoxideisthedominantfractionforPb.
The attenuation of heavy metals are are very complex processesinacidminedrainage.Furtherstudyisrequiredin thisregiontoanalysetheadsorptionandco-precipitationof heavy metals on other minerals such as aluminium and manganese minerals. A detailed study of the mineral transformationsintheaffectedareashouldalsobeinitiated.
ACKNOWLEDGEMENT
I take it to be proud privilege to avail this opportunity to expressmysincereanddeepsenseofgratitudetomymajor advisor Dr. Anupam Bhatnagar, Professor and Head, Department of Mining Engineering, College of Technology
and Engineering, MPUAT, Udaipur for this stimulating guidance, constructive suggestion, keen and sustained interestandincessantencouragementbestowedduringthe entire period of investigation, as well as critically going throughthemanuscript.
Iamgratifiedtorecordsincerethankstothememberofthe advisorycommitteeDr.S.C.Jain,Professor,Departmentof Mining Engineering, CTAE, Udaipur; Dr. Bheru Lal Salvi, AssociateProfessor,DepartmentofMechanicalEngineering, CTAE, Udaipur; Dr. Manjeet Singh, DRI Nominee and Assistant Professor, Department of Soil and Water ConservationEngineering,CTAE,Udaipurforthisgenerous gesturesandvaluablesuggestioninplanningandexecution ofthisstudy.
Withrespectanddeepsenseofgratitude,Iherebyexpress mythanktoDr.P.K.Singh,Dean,CollegeofTechnologyand Engineering, Udaipur for providing necessary facilities to carryoutmywork.
REFERENCES
[1] AllenSK,AllenJMandLucasS,1996.Concentrationsof contaminants in surface water samples collected in west-centralIndianaimpactedbyacidicminedrainage. EnvironmentalGeology,27(1):34–37.
[2] Johnson C A, 1986. The regulation of trace element concentrationsinriverandestuarinecontaminatedwith acid mine drainage: The adsorption of Cu and Zn on amorphous Fe oxyhydroxides. Geochimica et CosmochimicaActa,50(11):2433–2438.
[3] LeeG,BighamJMandFaureG,2002.Removaloftrace metals by coprecipitation with Fe, Al and Mn from naturalwaterscontaminatedwithacidminedrainagein the Ducktown Mining District, Tennessee. Applied Geochemistry,17(5):569–581.
[4] LeeJEandKimY,2008.Aquantitativeestimationofthe factorsaffectingpHchangesusingsimplegeochemical datafromacidminedrainage.EnvironmentalGeology, 55(1):65–75.
[5] Li Y T, Becquer T, Dai J, Quantin C and BenedettiMF, 2009. Ion activity and distribution of heavy metals in acid mine drainage polluted subtropical soils. EnvironmentalPollution,157(4):1249–1257.
[6] Motsi T, Rowson NA and Simmons MJH, 2009. Adsorptionofheavymetalsfromacidminedrainageby natural zeolite. International Journal of Mineral Processing,92:42–48.
[7] Ranville M, Rough D and Flegal A R, 2004. Metal attenuationattheabandonedSpencevillecoppermine. AppliedGeochemistry,19(5):803–815.
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page85
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056

Volume: 10 Issue: 1 | Jan 2023 www.irjet.net p-ISSN: 2395-0072
[8] Shikazono N, Zakir H M and Sudo Y, 2008. Zinc contaminationinriverwaterandsedimentsatTaisyu Zn-Pb mine area, Tsushima Island, Japan. Journal of GeochemicalExploration,98(3):80–88.
[9] Ullrich S M, Ramsey M H and Helios-Rybicka E, 1999. Totalandexchangeableconcentrationsofheavymetals in soils near Bytom, an area of Pb/Zn mining and smeltinginUpperSilesia,Poland.AppliedGeochemistry, 14(2):187–196.
[10] WuP,TangCY,LiuCQ,ZhuLJ,PeiTQandFengL J, 2009.GeochemicaldistributionandremovalofAs,Fe, Mn and Al in a surface water system affected by acid mine drainage at a coalfield in Southwestern China. EnvironmentalGeology,57(7):1457–1467.
© 2022, IRJET | Impact Factor value: 7.529 | ISO 9001:2008 Certified Journal | Page86
