Top 10 ASH Myeloma Research News
American Society of Hematology annual meeting highlights exciting myeloma research abstracts

The IMF International Myeloma Working Group and the IMF Leadership Team work to advance the field of myeloma at the premier forum for hematologists
ALSO IN THIS EDITION:
For the 19th year, the IMF Myeloma Voices Team of patient advocates attend ASH to foster connections between the patient and the scientific communities
The IMF Mourns the Passing of Dr. Brian G.M. Durie A visionary who changed the field of myeloma
The International Myeloma Foundation (IMF) mourns the passing of Dr. Brian G.M. Durie, co-founder of the organization and a visionary in the field of myeloma. A prolific myeloma researcher, Dr. Durie authored more than 700 research papers and, up until his death on October 12 at age 82, was energetically engaged in research projects worldwide. His 1975 pioneering work, the Durie-Salmon Staging System, was the first tool ever to classify patients with myeloma based on the correlation between the amount of myeloma and the damage caused by it.
Dr. Durie co-founded the IMF in 1990 together with Susie Durie and myeloma patient Brian D. Novis. The IMF became the first organization dedicated exclusively to myeloma that served the myeloma community through research, education, support, and advocacy. Dr. Durie stressed the importance of treating the individual, not just the disease. He empowered patients and inspired physicians. Dr. Durie made lifetime friends of patients and he served as a mentor to several generations of myeloma researchers the world over.

Brian G.M. Durie, MD (1942–2025)
To accelerate the search for a cure, Dr. Durie was instrumental in establishing the IMF Scientific Advisory Board (SAB) in 1995, the IMF International Myeloma Working Group® (IMWG®) in 2001, and the IMF Black Swan Research Initiative® (BSRI®) in 2012. The collaborative research by members of the IMWG has produced many published disease-treatment guidelines followed by doctors internationally. The BSRI-supported iStopMM® research project is shedding light on the earliest stage of myeloma by using the deep reserve of genetic data in Iceland.
“Dr. Durie was a giant in the field like no other. He leaves behind a legacy that will endure forever – from his incredible contributions to myeloma research to the superb care and counseling he provided patients; from educating almost all practicing myeloma experts today to helping advance patient care through important clinical trials; from developing the Durie-Salmon Staging System to co-founding the IMF, a patient-centered organization, which he led for three decades. He had an enormous impact –not just on myeloma but on all of oncology. I have lost a dear friend and mentor and will forever cherish his kindness,” said IMF Chairperson of the Board S. Vincent Rajkumar, MD (Mayo Clinic –Rochester, MN).
“I am deeply saddened by the passing of Dr. Brian G.M. Durie, whose groundbreaking research and unwavering dedication
transformed the understanding and treatment of myeloma. His leadership, compassion, and vision profoundly inspired all who had the privilege to know and work alongside him. His legacy will live through the countless lives he touched and the advances he made for patients worldwide,” said IMF Scientific Advisory Board Member Nikhil Munshi, MD (Dana-Farber Cancer Institute – Boston, MA).
“I had the privilege of being with him at an academic meeting in Rome the week before [his passing], with other myeloma experts. We enjoyed two very informative and productive days together. His worldwide contributions to research and care were, above all, patient-focused. Brian’s foundational role at the IMF reflected this as a central part of his vision along with his wife Susie and their great team,” said Paul G. Richardson, MD (Dana-Farber Cancer Institute – Boston, MA).
Dr. Durie was born in December 1942 in Gullane, Scotland. He graduated from the University of Edinburgh Medical School in 1966 and moved to the United States to complete his medical residency and subspecialty training at the Mayo Clinic in Minnesota. In 1989, he became a professor and Head of the Department of Clinical and Laboratory Hematology at the University of London, where he also established a myeloma program. In 1992, he returned to the United States and served as a myeloma specialist physician at Cedars-Sinai Medical Center in Los Angeles.
Dr. Durie’s dedication and contributions to medical science earned him numerous accolades, including the 2009 Waldenström Award for Myeloma Research and the 2014 Mayo Distinguished Alumni Award. In 2019, Dr. Durie and his wife Susie were awarded a joint Honorary Doctorate for Scientific Excellence from the Vrije Universiteit Brussel (VUB) in Belgium. Since 2018, the IMF has been bestowing the annual Brian G.M. Durie Outstanding Achievement Award to myeloma researchers in recognition of excellence.
Dr. Durie is survived by his wife, Susie Durie, his daughter, Annabel Reardon, and his son, Benjamin Durie, along with his four grandchildren. He will be enormously missed, but his legacy will undoubtedly continue. MT
At myeloma.org/honoring-brian-gm-durie-md , you can view the informative “Ask Dr. Durie” video series and read his blogs. We hope that Dr. Durie’s wisdom will bring you comfort as we mourn his passing and honor his legacy.
Dear Members of the Myeloma Community,
Thank you for your continued commitment and partnership. Your strength and determination inspire everyone at the IMF, and we are grateful to stand with you. Whether you are living with myeloma, caring for someone with myeloma, supporting research, or providing care, your efforts make a real difference and give purpose to our work.
In December 2025, the global myeloma community gathered at the American Society of Hematology (ASH) Annual Meeting in Orlando, Florida. The progress shared there was encouraging. One message was clear: research and treatment for myeloma are moving forward faster than ever. New treatment combinations, earlier use of targeted and cellular therapies, and many promising treatments in development are improving both how long and how well people live with myeloma.
ASH also demonstrated the importance of collaboration. Researchers, clinicians, people living with myeloma, and advocates are working together to move science forward. The advances shared in Orlando reflect years of hard work and strengthen our belief that myeloma is more manageable today – and that continued innovation brings us closer to a cure. While challenges remain, the pace of progress gives us real reason for hope.
As science advances, our commitment to you stays the same. At every stage, the IMF continues to offer trusted education, timely resources, and compassionate support for people with myeloma and their care partners. Whether someone is newly diagnosed, facing a relapse, managing the disease long-term, or living in survivorship, we aim to provide clear information and meaningful support. Through education, support services, and connections with others who understand the experience, we help people make informed decisions and feel less alone.
Care partners are a vital part of the myeloma community. We recognize the important role they play, as well as the emotional, educational, and practical support they need. By offering resources designed specifically for care partners, we work to strengthen the support around every person living with myeloma.

We also remain deeply committed to research. Guided by our Scientific Advisory Board of leading myeloma experts from around the world, the IMF continues to explore new ways to support research that helps people live well with myeloma. We will also continue to lead the IMF’s International Myeloma Working Group, bringing together global experts to advance research, guide care, and improve lives. This work is driven by you – your experiences, your participation in research, and your belief in what is possible.
Looking ahead, we are hopeful. Hopeful because of the remarkable progress in science and treatment. Hopeful because of the strength of this community. And hopeful because together, we are changing what it means to live well with myeloma.
Thank you for your trust and continued partnership. We are honored to serve you and remain committed to supporting you today while working toward a better tomorrow.
With gratitude and hope,
Heather Cooper Ortner

Top 10 Abstracts from the Meeting of
By Dr. Joseph Mikhael IMF Chief Medical Officer
Nearly 30,000 individuals who are part of the blood diseases community gather every December at the annual meeting and exposition of the American Society of Hematology (ASH) to present and discuss the most significant recent data in research. This year, more than 9,000 research abstracts were submitted to ASH for consideration and more than 8,000 abstracts were selected for presentation. Remarkably, more than 1,500 of the abstracts presented were in multiple myeloma!
Myeloma continues to be the fastest-growing topic at the ASH meetings. It is so encouraging to see the progress we have made in myeloma research that holds great promise for our patients and their families. Below, I share my personal Top 10 myeloma-related abstracts, divided into two groups. Five abstracts are related to chimeric antigen receptor (CAR) T-cell therapy and five are related to bispecific antibody therapies.
CAR T-cell therapy
Taken together, these following five abstracts provide genuine hope for us to provide CAR T-cell therapy to our patients more easily, more effectively, and more safely.
LBA-1:
in vivo CAR-T
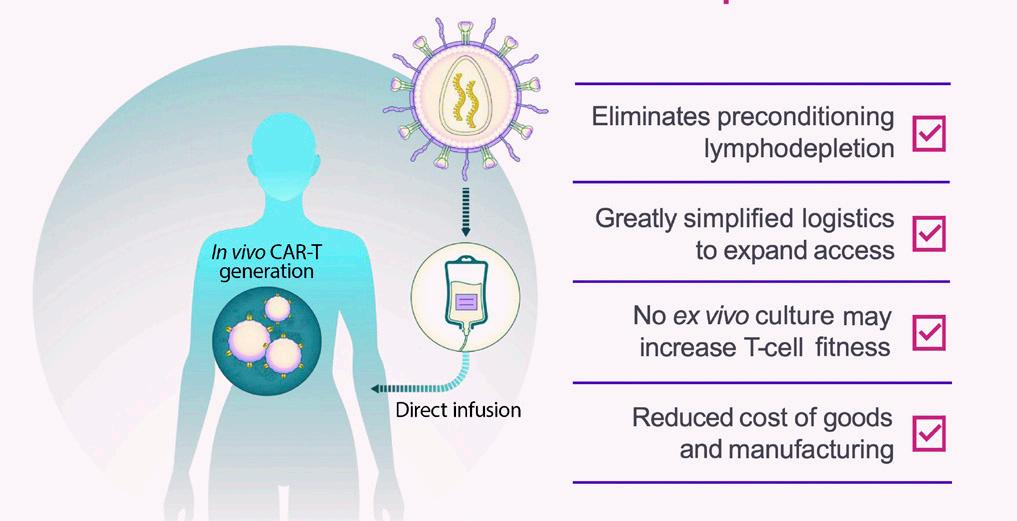
This “late breaking” abstract (LBA) was one of only six abstracts that featured recently discovered results. It reported on the first three patients treated with in vivo CAR-T, meaning that the process takes place inside a patient. Typically, CAR-T is done ex vivo, which means we remove T cells from a patient’s bloodstream, then modify them in the laboratory and re-infuse the engineered T cells into the patient. The new in vivo CAR-T drug is delivered directly to the patient, converting the patient’s T cells to CAR-T cells inside their bodies, with no need to collect, engineer, manufacture, and then re-infuse the CAR-T cells into the patient.
In vivo CAR-T is an amazing concept. It could allow much easier access to CAR T-cell therapy, which currently is a challenge and a huge disparity in myeloma. The patients treated with in vivo CAR-T in the clinical trial, all of whom have high-risk multiple myeloma (HRMM) and had been heavily pre-treated, achieved MRD-negativity in the first month and experienced fewer side effects than we typically see in ex vivo CAR T-cell therapy. In vivo CAR T-cell therapy is a big step forward in making the most effective myeloma therapy safer and more accessible for our patients.
Abstract 94:
Long-term follow-up of standard-risk patients treated with Carvykti Carvykti® (ciltacabtagene autoleucel, “cilta-cel”) is the CAR T-cell therapy currently used as early as first relapse in myeloma (second-line therapy). Two important clinical trials evaluated its use in patients: the CARTITUDE-1 study of patients with over 4 (on average 6) lines of therapy and the CARTITUDE-4 study of patients with 1 to 3 prior lines of therapy. The long-term follow-up of the standard-risk patients in those studies demonstrated outcomes we have never seen before.
In early relapse, the progression-free survival (PFS) at 30 months was 80%. In late relapse, the PFS was 60%. Historically, CAR-T was used preferentially in high-risk patients, but now we see very impressive results in standard-risk patients with unprecedented length of time in remission. The CARTITUDE study also provides us more specific data to share with standard-risk patients when making a decision about CAR T-cell therapy.
Abstract 1034:
Enhancing the safety of Carvykti
One of the challenges we face with Carvykti is the delayed neurological side effects, neurotoxicity that typically happens a month or more after treatment. It can be quite severe, such as Parkinson-like and Guillain-Barré-like syndromes. Preventing or reducing these rare neurotoxicities is important as we seek to deliver highly effective treatment more safely.
This study evaluated more than 750 patients treated with Carvykti and arrived at two important conclusions. Firstly, we can reduce the risk of neurotoxicities by giving effective “bridging therapy,” the treatment we give after T cells are collected but before they are re-infused. Secondly, a rise in the absolute lymphocyte count (ALC) may predict a higher risk of neurotoxicity, and strategies to reduce the ALC may be of value.
the American Society of Hematology
Abstract 258:
Dual-Targeting BCMA and CD19 FasTCAR-T
As we seek to make CAR T-cell therapy even more effective, a CAR-T that targets two antigens on the surface of myeloma cells is designed to be more specific in adhering to myeloma cells. This is explored in the molecule GC012F/AZD0120, which was also tested in patients with newly diagnosed multiple myeloma (NDMM). This was highly effective, with 100% of 30 patients responding, and with no cases of delayed neurotoxicity. We need more time and data, but this could signal even more effective and safe CAR T-cell therapy in the future.
Abstract 256:
A novel CAR-T with anito-cel
In another approach to improving the efficacy and safety of CAR T-cell therapy, the new anito-cel molecule was tested in patients with relapsed myeloma. Anito-cel is unique in its novel binding of the CAR-T to the myeloma cell, using “D-domain” binding that might be more precise. Nearly all patients in this study responded with an overall response rate (ORR) of 97%, and there have been no cases of delayed neurotoxicity with more than a year of follow-ups of over 100 study patients. Having a highly effective CAR T-cell therapy with minimal or no risk of delayed neurotoxicity is highly desirable.
Bispecific antibodies
The following abstracts clearly show that there is much more to come for the use of bispecific antibodies in myeloma. These studies of bispecific antibodies include using them in combination, treating patients earlier in the disease course, as consolidation, and with MRD guidance to deepen responses.
LBA-6:
Tecvayli and Darzalex in early
relapse
The highly anticipated late-breaking abstract from the MajesTEC-3 phase III clinical trial, which compares the combination of the bispecific antibody Tecvayli® (teclistamab-cqyv) plus the monoclonal antibody Darzalex® (daratumumab) to the standard approach with a triplet (3-drug) combination in patients with 1 to 3 prior lines of therapy.
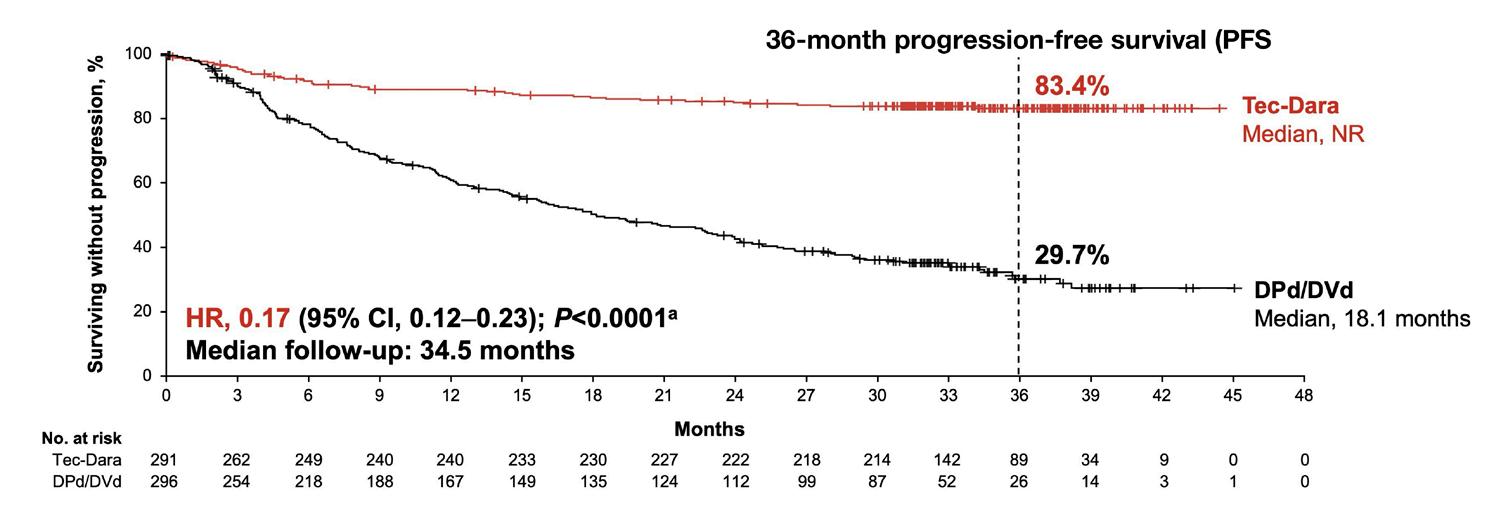
LBA-6 may be the most talked about abstract in myeloma; the results are genuinely dramatic. At the 3-year mark, 83% of patients on the Tecvayli + Darzalex combination were still in remission, whereas only 30% were in remission on a triplet. The overall survival (OS) was also improved at the 3-year mark (83% vs 65%). Furthermore, patients were treated with less Tecvayli than historically used, as they matched it to the dosing of Darzalex: weekly for 8 weeks, then every other week for 16 weeks, then once every 4 weeks.
There was, however, a concerning infectious signal, as initially 4% of patients died of an infection. But the study investigators altered the protocol to ensure more infection precautions, such as the use of intravenous immunoglobulin (IVIG). Thereafter, significantly fewer infections occurred. The data from this study will almost definitely lead to the use of bispecific antibodies earlier in the disease course. Bispecific antibodies are currently approved for use in myeloma only after 4 lines of therapy.
Abstract 367:
Tecvayli and Darzalex in frontline therapy
The same combination of Tecvayli + Darzalex was tested in frontline therapy in newly diagnosed patients who are not eligible for transplant. This was part of a French trial that combined Tecvayli
(continues on next page)



plus the immunomodulatory agent Revlimid® (lenalidomide) in one cohort (group of patients) and Tecvayli + Darzalex in another cohort. Although it was only used in 37 patients, the response rate was 100% and no patients have yet relapsed at 10 months of follow-up! This is an important combination to watch as it may lead to the frontline use of bispecific antibodies, and challenging our current use of triplet and quadruplet (4-drug) combination therapies.
Abstract 248:
Lynozyfic to achieve MRD-negativity in frontline therapy
I am including this abstract in my Top 10 list because of the novel design of this clinical trial. The study evaluated myeloma patients who had standard induction therapy (initial phase of treatment) to achieve remission but still remained positive for minimal residual disease (MRD). Patients received 4 cycles of the bispecific antibody Lynozyfic™ (linvoseltamab-gcpt) to see if they could deepen response to MRD-negativity – and indeed every patient did.
The importance of MRD status has been well established. To deepen response to treatment, the unique approach of this study added a bispecific antibody that targets the B-cell maturation antigen (BCMA), a protein found on the surface of myeloma cells. This form of treatment is currently not used in induction. This study is important as it widens the potential use of bispecific antibodies, uses MRD to guide therapy, and provides another mechanism of action to control myeloma.
Abstract 699:
Cevostamab consolidation after CAR T-cell therapy
One potential method to make CAR T-cell therapy more effective is to follow it with a bispecific antibody that has a different target than the CAR T-cell therapy. Currently, CAR T-cell therapy targets the BCMA antigen on the surface of the myeloma cell. Cevostamab is a new bispecific antibody that is being developed to target a different antigen, FcRH5.
Patients in this study received CAR T-cell therapy, followed by cevostamab for approximately 6 months. At this point, the study stopped treatment in patients who were MRD-negative patients but continued treatment of MRD-positive patients. Full results are pending. The study design is fascinating as it aims to extend the length of benefit from CAR T-cell therapy with a new bispecific antibody that uses a different target on the myeloma cell. Guided by MRD, this could become part of the future of CAR T-cell therapy in myeloma.
Abstract 698:
Talvey plus Tecvayli in patients with EMD
One of the most challenging forms of myeloma is when the disease grows outside of the bone marrow. This is known as extra-medullary disease (EMD). Historically, myeloma patients with EMD had lower response rates to treatment and, in particular, had very short periods of remission as the disease grows aggressively. This study combines two different bispecific antibodies that have different targets on the myeloma cell. Talvey® (talquetamab) targets GPRC5D and Tecvayli targets BCMA.
Study data demonstrated remarkable results with a response rate of 80%, nearly doubling prior therapies and lasting on average more than 12 months. This study may also lead to a novel use of bispecific antibodies by combining them together, especially in challenging-to-treat forms of myeloma.
In conclusion
These are truly exciting times in myeloma research, yielding more effective and safe therapies for our patients, and providing genuine hope as we continue to move closer to a cure. MT
STAY INFORMED! Contact the IMF InfoLine with your myeloma-related questions and concerns. Phone lines are open 9 a.m. to 4 p.m. (Pacific) Monday through Friday at 1.818.487.7455 or you can email us at InfoLine@myeloma.org or visit mmsm.link/infoline to schedule a convenient time to talk with an IMF InfoLine Coordinator.
Myeloma Support Group Leaders at ASH
By Robin Tuohy IMF Vice President, Support Groups
The 67th American Society of Hematology (ASH) Annual Meeting and Exposition is the world’s most comprehensive gathering for hematology professionals and the premier global forum for the latest scientific and clinical advances in blood disorders. On December 6–9, 2025, ASH welcomed approximately 31,000 attendees from around the world. The meeting was held in Orlando, Florida. Underscoring the remarkable pace of progress in myeloma, ASH featured more than 1,500 abstracts focused on this disease.
For 19 years, the International Myeloma Foundation (IMF) has invited support group leaders to participate in ASH through the Myeloma Voices program. This patient-led initiative serves two essential purposes: providing leaders with firsthand access to the latest myeloma research and clinical trial updates, and elevating patient and care partner perspectives within the scientific community at ASH. These voices then ripple outward – locally through support groups, globally through social media, and across the broader myeloma community – strengthening engagement and connection at every level.
The Myeloma Voices team at ASH 2025 included 11 support group leaders attending in person plus 4 leaders participating virtually. Each participant committed to attending scientific sessions, reviewing poster presentations, and sharing insights using patient-friendly language and their own unique voice. In combination with the IMF’s interviews with leading myeloma experts, the Myeloma Voices program provided a comprehensive and accessible view of the depth and breadth of ASH for our CoMMunity. Through local support groups, community
outreach, traditional media, blogs, and social platforms like X (formerly Twitter), Facebook, and LinkedIn, the team helped ensure that timely research updates reached myeloma patients and families worldwide.
Preparation for the program began months before the meeting. Myeloma Voices team members represent the diverse perspectives of myeloma patients, care partners, healthcare professionals, and advocates. Some participants were newly navigating their myeloma diagnosis, while others were long-term survivors and seasoned advocates. Their professional backgrounds included science, academia, nursing, and patient advocacy, all of which enhanced the team’s ability to understand complex information and communicate it with both clarity and compassion.
The pace of ASH is highly demanding, with days often beginning before sunrise and extending late into the evening. Conference schedules frequently exceeded 12 hours of educational sessions in a single day. Each morning, the in-person members of the Myeloma Voices team coordinated attendance of simultaneous myeloma presentations to ensure the broadest possible coverage. Breakfasts, lunches, and dinners became working meetings, including conversations with industry partners and donors. Each interaction was an opportunity for myeloma support group leaders to advocate for patient-centered and care partner–centered priorities. In brief moments between sessions, the team recorded videos and shared social media updates to highlight key abstracts in real time.
Teresa Miceli, BSN, RN (Member, IMF Nurse Leadership Board) was part of our in-person Myeloma Voices team and served as an invaluable resource, helping translate complex
(continues on next page)

medical concepts and guide discussions. Meanwhile, the virtual Myeloma Voices team met daily to review key abstracts, support one another in understanding scientific data, and maintain momentum throughout the conference.
In-person participants were also honored to attend the annual IMF’s International Myeloma Working Group (IMWG) breakfast meeting. This standing-room-only gathering of more than 200 global myeloma experts is a highlight of ASH each year. The IMWG breakfast at ASH is an exclusive forum of collaboration among IMWG members that offers deep insight into some of the most impactful studies to be presented at ASH.
IMF Chairperson of the Board, Dr. S. Vincent Rajkumar, opened the IMWG breakfast with a moment of silence honoring IMF co-founder Dr. Brian G. M. Durie. It was a deeply emotional and humbling moment to witness an entire room of myeloma researchers stand in unison to pay tribute to a man who made our myeloma world profoundly better.
For the third consecutive year, the Myeloma Voices program concluded with a Facebook Live event featuring Dr. Joseph Mikhael (IMF Chief Medical Officer). During this interactive session, Dr. Mikhael summarized the most impactful myeloma research presented at ASH, while Myeloma Voices leaders shared questions and reflections shaped by their lived experiences. Members of the broader myeloma CoMMunity joined the conversation through live chat, reinforcing the program’s role in connecting scientists, clinicians, patients, and care partners. The Facebook Live event concluded with a special surprise appearance by IMF’s new President and CEO, Heather Cooper Ortner, who thanked participants for their engagement and spoke to the bright future ahead.

Throughout the meeting, Myeloma Voices leaders captured their experiences in real time – reflecting on scientific sessions, meaningful conversations, and the personal impact of having patients and care partners at ASH. Their words offer a powerful glimpse into the energy, dedication, and heart behind the Myeloma Voices program.
I encourage you to explore these perspectives further on the next page of Myeloma Today and by visiting the Myeloma Voices at ASH webpage at ash2025blogs.myeloma.org for patient-friendly summaries, videos, and reflections.
The Myeloma Voices at ASH program is generously supported by donors and by industry partners. The IMF extends our sincere thanks to AbbVie, GSK, J&J, Karyopharm Therapeutics, Regeneron, Sanofi, and others. We are deeply grateful for your continued partnership.
I would like to thank the entire IMF staff who help make the Myeloma Voices program possible, and especially the IMF Support Group Team for their dedication in planning and executing this annual event. Finally, it is with heartfelt respect and gratitude that I thank each of the Myeloma Voices support group leaders who give so generously their time and energy, immersing themselves in ASH and sharing their voices on behalf of our CoMMunity. MT
Please contact Robin Tuohy at rtuohy@myeloma.org or visit myeloma.org/support-groups for information about joining a local myeloma support group or launching a new one in your area.

Insights from the Myeloma Voices Team at ASH
“Attending ASH gives me more confidence as a myeloma advocate and support group leader. Seeing how the doctors really are working together to find a cure for myeloma, being there when the abstracts are presented, and hearing questions posed by one specialist to another (which can be similar to my questions), that’s all valuable to me.”
– Sheri Baker, diagnosed in 2011
“The most important part of attending ASH was the strong sense of community that came together: healthcare professionals, patients, researchers, and scientists. Community is essential for health and wellbeing, resilience and empowerment, skill-building and resource-sharing, and working toward a common goal. All of these were clearly evident at ASH.”
– Mindy Fast, diagnosed in 2007
“It’s so important to have myeloma patients attend ASH. It isn’t just that ASH is where data is shared, it’s where lives of patients are shaped. We can help turn the data into meaningful information for our patients and extend the HOPE we feel to reach our patient communities. We take the science back to our communities and we bring the voice of our communities to the scientists.”
– Terry Glassman, diagnosed in 2022
“Communicating awareness is my major objective. Then I want people to understand that a myeloma diagnosis is not a death sentence. Current medications offer patients a better quality of life than in years past. The progress of myeloma treatment is rapid. Newly diagnosed patients can achieve remission status sooner. They can be hopeful for living a longer, fulfilled lifetime with myeloma.”
– Diane Hunter, diagnosed in 2017

“The #ASH25 meeting was another banner event. This ASH meeting has left me more hopeful than any other, which is saying a lot. There are too many highlights to list. In general, the outcomes data is jaw-dropping. As Dr. Mateos stated in a slide of her presentation, ‘Greatest PFS treatment effect to date (HR, 0.17), with plateauing curve after ~6 months suggests potential for a functional cure.’ Mic drop!”
– Teresa Miceli, myeloma nurse
“The opportunity to network and to meet with industry partners was a vital part of this experience. What stood out is the impression that they genuinely valued feedback from patients, which makes me feel important and reinforces the critical role myeloma advocates play in the development of patient-centric therapies.”
– Rose Simon, diagnosed in 2023
“As a person living with myeloma for 25 years, plus as a volunteer patient advocate, I speak with myeloma patients quite often. Attending ASH provides me with the key updates and resources specific to myeloma that I can share to help others with their own conversations with their healthcare team. Hope in Research and Science! “
– Michael Tuohy, diagnosed in 2000
“There’s so much work going into myeloma to help patients like me! They are working on every phase of this disease, from MGUS to relapsed myeloma. Multiple lines of therapy, novel approaches, CAR T-cell therapies, bispecific antibodies, TRIspecifics… I’m excited to learn and to share it.”
– Eric Wolf, diagnosed in 2012

Replay the webinar video and view the presentation slides by visiting videos.myeloma.org and clicking on the ASH
IMF Asian Myeloma Network Holds
By Daniel Navid IMF Senior Vice President, Global Affairs
The 2025 annual meetings of the IMF Asian Myeloma Network (AMN) were held on October 16–19 in Nagoya, Japan. Once again, the AMN meetings were the largest and most ambitious regional activities to date, with myeloma specialists, patients, and care partners coming together from across Asia.
The 5th AMN Patient Forum
On October 16, myeloma patient groups from across Asia came together to share and learn from their individual experiences. In attendance were 82 representatives from patient groups in China, Hong Kong, India, Japan, Korea, Malaysia, Philippines, Singapore, Taiwan, and Thailand. As in past years, the AMN Patient Forum was chaired by AMN Executive Member Dr. Daryl Tan of Raffles Cancer Center, Raffles Hospital in Singapore.
This year the Patient Forum benefited from a presentation by a government official, Dr. Daisaku Sato, Councilor of the Japan Ministry of Health, Labour and Welfare. Dr. Sato explained the streamlined procedures being adopted for drug approval in Japan and paid tribute to the work of the Japan Myeloma Patient Society in helping to promote improved access. He also pledged to help facilitate Japanese involvement in AMN clinical trial projects.
Presentations were made by Diane Moran (IMF Interim CEO and Senior Vice President of Strategic Planning) on IMF patient support activities and by Kyoko Joko on the extensive work of the Japan Myeloma Patient society, the oldest such group in Asia. The Patient Society Reports were led by Dr. Tan. It was satisfying to see the continued growth by the newer patient societies in Malaysia, Philippines, Taiwan, and Thailand. Impressive progress was demonstrated by the more established groups in China, Hong Kong, Japan, Korea, and Singapore. For the first time, a report was provided from India, where several patient groups are cooperating with the Indian myeloma physician group (IMAGe).
Lively discussions followed in sessions of the Working Groups on patient empowerment, what to expect from a support group, how to work with physicians, and fundraising. Participants exchanged information and identified follow-up project requirements, which are being pursued.
The AMN Patient Forum was supported by industry partners, including Bristol Myers Squibb, Johnson & Johnson, Pfizer, and Sanofi, as well as Chinese companies BeOne, J&J-China, Pfizer-China, Sanofi-China, and Takeda-China.
The 9th AMN Summit
On October 17–19, the 9th AMN Annual Summit was Asia’s largest gathering ever of myeloma specialists, with 186 participants from all 11 countries and regions of the AMN: China, Hong Kong, India, Japan, Korea, Malaysia, Philippines, Singapore, Taiwan, Thailand, and Vietnam. This year, the AMN Summit was expanded by one additional day to allow for more sessions and in-depth discussions. The Summit was co-chaired by AMN Chair Dr. Wee Joo Chng of Singapore and AMN Executive Member Dr. Shinsuke Iida of Japan.
The Summit keynote address was delivered by AMN International Expert, Dr. Andrew Spencer of Australia on “Blood Based Diagnosis and Monitoring.” Sessions that followed covered Optimal Frontline Treatment of Multiple Myeloma, Mass Spectrometry, Immune Therapy, Novel Treatments, and Other Plasma Cell Disorders.
The second day of the Summit began with a breakfast session of the newly formed AMN Young Investigators Committee, a group of 21 leading young Asian hematologists representing all 11 countries and regions of the AMN under the leadership of AMN Deputy Chair, Dr. Hiroshi Handa of Japan. This committee gathers feedback from younger members into the work of the AMN. Several project ideas were identified to be pursued.
In addition, four Working Groups were convened: Infection and Side Effects (led by Dr. James Chim of Hong Kong and Dr. Jeffrey Huang of Taiwan), Immunotherapy (led by Dr. Iida and Dr. Juan Du of China), New AMN Research (led by Dr. Kihyun Kim of Korea and Dr. Chng), and Resource Stratified Guidelines (led by Dr. Tan and Dr. Uday Yanamandra of India). Consensus guidelines as well as several new AMN projects will be produced in follow-up to these sessions.


Annual Meetings in Nagoya, Japan
The Summit also reviewed the status of AMN Clinical Trials led by Dr. Chandramouli Nagarajan of Singapore and Dr. Dok Hyun Yoon of Korea, as well as the Status of the AMN Tissue Bank led by Dr. Chng and Dr. Handa.
The final session of the AMN Summit considered future directions for membership, outreach, and cooperation with other regions, as well as future plans for the AMN Summit, AMN Master Class, AMN Patient Forum, and AMN clinical trials. The 10th AMN Summit and 6th Patient Forum will be held in Shanghai on October 15–18, 2026. The 6th AMN Master Class will be held in Singapore on July 2–5, 2026.
The AMN Summit was supported by industry partners. Platinum level: Bristol Myers Squibb, Glaxo Smith Kline, and Sanofi; Gold level: Binding Site, Johnson & Johnson, and Pfizer; Silver level: AbbVie, IASO, Regeneron, and Sebia; Local level: Becton Dickenson, Chugai and ScBio, as well as Chinese companies BeOne, J&J China, Pfizer-China, Sanofi-China, and Takeda-China.
2025 AMN Distinguished Service Award
Award… Since dedicating my research to myeloma in 2004, I have witnessed remarkable advances in treatments for the disease. The establishment of the AMN has been pivotal to progress in our region. Through the AMN, myeloma patients in Asia can access cutting-edge treatments. The AMN is a vibrant community of international colleagues and friends, united by our shared commitment to advancing myeloma care. I am most privileged and honored to participate in this transformative journey,” said Dr. Chim.
“I have known Dr. Chim for many years. He has contributed greatly to our understanding of myeloma. I thank and congratulate him for his service to the field,” said IMF Chairperson of the Board Dr. S. Vincent Rajkumar. “Dr. Chim has contributed so much to the development of myeloma care and research in Asia. We are so grateful for all he has done. He is truly deserving of this award,” noted AMN Chairman Dr. Wee Joo Chng.
In closing

Dr. James Chim is the recipient of the 2025 AMN Distinguished Service Award, established in 2023 to recognize and honor the lifelong achievements of myeloma specialists who have made a significant impact in the field of research, clinical trials, and patient support. Dr. Chim is a world-renowned myeloma expert, a member of the IMF International Myeloma Working Group (IMWG), a founding member of the AMN, the founder and current chair of the Hong Kong Society of Myeloma, and the founder of Hong Kong Myeloma Care and Share patient group.
“I am deeply honored, profoundly humbled, and truly grateful to receive this year’s Asian Myeloma Network Distinguished Service
The AMN Summit and the AMN Patient Forum have been extremely successful, generating a lasting impact not just within the Asian region but across the global myeloma community. The IMF extends its warmest gratitude to all AMN members and Patient Forum attendees, and to our industry partners. The AMN is also deeply grateful to the international experts who helped guide the work of the Summit: Dr. Shaji Kumar, Dr. Tom Martin, Dr. Mohamad Mohty, and Dr. Andrew Spencer. The AMN also thanks the IMF team along with Jinsil Lee of Lotte/JTB and Minako Unno, Rita Li and Ryohei Asano of Destination Asia, and volunteers from the Nagoya Convention Bureau, all of whom ensured the smooth running of the events. MT
Visit amn.myeloma.org to learn more about AMN members, events, and activities.

2025 IMF LAMN Master Class & Summit
By Dr. Joseph Mikhael IMF Chief Medical Officer
The IMF was established in 1990 and began its global activities shortly thereafter. The IMF is dedicated to serving the myeloma community – patients, care partners, scientists, clinicians, and others – regardless of where they are located.
In 1995, the IMF established its first Scientific Advisory Board (SAB), comprised of myeloma specialists from around the world. At that time, the number of experts was small – in the two digits – and the IMF’s SAB included them all. Since then, the field of myeloma has grown immeasurably, and so has the IMF.
Currently, the IMF International Myeloma Working Group (IMWG), our key collaborative research arm, includes more than 360 members. The IMF Asian Myeloma Network (AMN), established in 2011, is bringing together myeloma experts from more than 10 countries to conduct clinical trials, create databases, and provide resources to both doctors and patients in the region.
Our IMF Latin America (IMF-LA) affiliate, based in Brazil, was founded in 2004 to provide resources, education, and support for myeloma patients and the medical community in South America. In 2024, the IMF launched the Latin American Myeloma Network (LAMN), patterned after the successful model of the AMN. The inaugural LAMN Summit was held in September 2024 in Rio de Janeiro, Brazil.
The incidence of myeloma in Latin America is becoming more prevalent, and this has become a concerning issue due to poorer outcomes. The LAMN was formed to help patients with access to novel therapies, increase the expertise in myeloma in the region, facilitate collaborative research, conduct clinical trials, generate local data, and educate the myeloma patient community. LAMN founding countries include Argentina, Brazil, Chile, and Uruguay. More recent additions include Colombia and Mexico.
In November 2025, the LAMN held a Master Class and Summit in Santiago, Chile. More than 30 individuals gathered to deepen their knowledge of myeloma, discuss research projects and clinical trials, and plan educational and outreach activities.
Held on November 14th, the Master Class covered the spectrum of myeloma from precursor conditions to frontline therapy, maintenance, early and late relapse, and how to deliver


immunotherapies more safely and effectively. Nearly all senior physicians from the region gave presentations, followed by extensive discussion. Between these lectures, junior faculty presented clinical cases, each leading to a detailed exchange about practical clinical management.
The LAMN Summit took place on November 15th, with project proposals presented by junior faculty. Seven projects were pre-selected from 20 that were submitted earlier in the year. Designed as collaborative projects across multiple centers, proposals included lab-based studies, surveys, clinical trials, and chart reviews. The scientific level of the proposals was commendable.
Next, attention turned to the upcoming launch of the first LAMN clinical trial, as well as potential future studies, with special emphasis on novel therapies such as antibody-drug conjugates (ADC), bispecific antibodies, and CAR T-cell therapy. The IMF team is excited to develop a deeper collaboration with our colleagues in Latin America, and we look forward to bringing the expertise from this region to the global programs of the IMF, including the IMWG.
The level of engagement and camaraderie across the two days of the LAMN Master Class & Summit demonstrated a strong sense of optimism for what the LAMN can accomplish in the region. I look forward to sharing with you the future activities and achievements of this important initiative. MT
Follow the growth and activities of the LAMN by visiting lamn.myeloma.org or email TheIMF@myeloma.org for more information.
YOU Can Help Break Down Barriers to Care
By Danielle Doheny IMF Director, Public Policy & Advocacy
Extraordinary advances in the treatment of myeloma have been made in the past decade. Yet many patients with myeloma continue to face persistent barriers that shape their care long before clinical decisions are made. We invite YOU to share your experiences with the IMF Advocacy Team as we work to ensure timely, affordable, and equitable access to care.
Barrier 1
Access to specialists and comprehensive care
Myeloma is a complex disease. Treatment outcomes are often better for patients who see a myeloma specialist, but access to a specialist is far from guaranteed. Patients may face insurance networks that restrict access or may need to travel long distances for care. This can be overwhelming for older patients, those living in rural areas, or individuals managing multiple health conditions.
Access to myeloma specialists, whether in person or virtually, remains a foundational issue. Telehealth is helping some patients consult with specialists online without extensive travel. However, telehealth access remains uneven and ongoing policy discussions will determine whether it will continue to be available and affordable for patients who rely on it. The IMF is a leader in shaping these policies, and we are working to ensure that legislators understand the importance of patient access to a specialist.
Barrier 2
Insurance design, cost-sharing, oral parity
Effective treatment of myeloma might not be affordable even for patients with insurance, who are faced with high cost-sharing that can quickly escalate to thousands of dollars out-of-pocket. Financial burdens affect a patient’s daily life and, for some, may determine whether they can stay on treatment.
Intravenous treatments are usually covered under an insurance plan’s medical benefit. Oral therapies often fall under the pharmacy benefit, where deductibles and coinsurance can be substantially higher. However, oral therapies are just as essential – and are often the standard of care – for many cancers, including myeloma. The IMF has a long history of leading federal efforts to address this barrier, advocating for oral parity laws and the Cancer Drug Parity Act to ensure that oral and infused treatments are covered equitably.
While progress has been made, inconsistencies remain, leaving many patients exposed to high costs that are unrelated to their medical need. When coverage decisions shape treatment choices, patients bear the consequences, not just financially, but in their quality of care. The IMF supports policies like the Safe Step Act, and has trained many patients who are experiencing this barrier to share their stories effectively with lawmakers.

Barrier 3
Utilization management and delays in care
Utilization management aims to control costs but is frequently applied without adequate consideration of complex cancers like myeloma. Requirements for prior authorization, step therapy, and non-medical switching can disrupt or delay care. Such hurdles can force patients to wait for approval, repeat therapies that are no longer appropriate, or switch medications for non-clinical reasons.
Myeloma therapies often require timely adjustments based on response to treatment, disease progression, or side effects. Delays can worsen treatment outcomes and erode trust between patients and their care teams. Advocacy is critical to ensuring that policies include transparency, appropriate safeguards, and expedited processes for serious and life-threatening conditions.
Why advocacy still matters
Advocacy is vital to ensuring that patients can access the care they need. Advances in myeloma therapies should reach every patient who needs them, when they need them, and without unnecessary hardship. Progress requires continued attention, engagement, and action. The IMF is working to remove all barriers to care, and we need YOU to join us.
Patients like YOU are stepping forward to be trained and equipped by the IMF to become grassroots advocates who can help shape policy by sharing their personal stories with lawmakers. The more stories the IMF can amplify, the more powerful our community’s collective voice becomes. MT
Reach out to us at advocacy@myeloma.org, visit advocacy.myeloma.org to learn more about our activities and how we support our advocates, and subscribe to the IMF Advocacy Newsletter at subscribe.myeloma.org





Myeloma Research at ASH 2025

P. Joy Ho, MBBS, DPhil
Royal Prince Alfred Hospital University of Sydney Sydney, Australia
Abstract LBA-1
INTERNATIONAL MYELOMA FOUNDATION
Founders
Brian D. Novis • Susie Durie • Brian G.M. Durie, MD
Board of Directors
Chairperson S. Vincent Rajkumar, MD
Christine Battistini
Loraine Alterman Boyle
Martine Elias, MSc
George T. Hayum
Jason Katz
Andrew Kuzneski, III
Sagar Lonial, MD

Salomon Manier, MD, PhD
Lille University Hospital Lille, France
Abstract 367
Nikhil Munshi, MD
Charles Newman, MS
Kent Oliver
Poornima Parameswaran, PhD
Matthew Robinson, MBA
Sanjay Singh
Maria Whitman, MBA
IMF Executive Team
In Vivo CAR T-cell Therapy for Myeloma: Revolutionary off-the-shelf treatment
Combination of Tecvayli and Darzalex: Unprecedented efficacy demonstrated in NDMM elderly transplant ineligible patients

Abstract LBA-6

María-Victoria Mateos, MD, PhD University of Salamanca Hospital Salamanca, Spain
MajesTEC-3 Phase III Clinical Trial: The combination of Tecvayli and Darzalex shows transformative results in early relapse
Surbhi Sidana, MD Stanford University School of Medicine Stanford, California, USA
Abstract 1034
Carvykti Safety Enhancement: Identification of potentially modifiable risk factors for delayed neurotoxicity
Watch the IMF’s interviews with abstract authors Go to videos.myeloma.org and click the ASH tab.
Heather Cooper Ortner President & Chief Executive Officer
Peter Anton Vice President, Marketing
Joseph R. Mikhael, MD Chief Medical Officer
Diane Moran Senior Vice President, Strategic Planning
Elizabeth Ableson
Administrative Assistant, Human Resources
Nikki Arends Senior Planner, Meetings & Events
Betty Arevalo Inventory Control Manager
Katie Atkins Associate Director, Support Groups
Becky Bosley Director, Support Groups
Brittnay Brandon
Senior Coordinator, Meetings & Events
Michelle Carroll Director, Prospect Development
Lisette Contreras
Administrative Assistant, Meetings & Events
Danielle Doheny Director, Public Policy & Advocacy
Jon Fitzpatrick Senior Manager, Meetings & Events
Esther Garnica Administrative Assistant, Operations
Simona Grace Director, Development
Sherrie Guerrero
Senior Director, Human Resources
Paul Hewitt Coordinator, InfoLine
Kevin Huynh Coordinator, Tech Solutions
Marya Kazakova
Senior Director, Editor-in-Chief, Publications
Missy Klepetar Coordinator, InfoLine
Sapna Kumar Marketing Strategist
Lisa Paik
Executive Vice President, Research & Operations
Jennifer Scarne Chief Financial Officer
Robin Tuohy Vice President, Patient Support
Phil Lange Director, Accounting
Jason London
Senior Manager, Marketing & Communications
Kristina Mease Director, Meetings & Events
Marquela Nastri
Grants Specialist
Jim Needham
Publication Design
Meghan O’Connor
Meeting & Project Manager, Content & Communications
Selma Plascencia Senior Director, Operations
Joy Riznikove
Database Administrator
Cecilia Romero Project & Technology Manager, Support Groups
Miko Santos Senior Manager, Tech Solutions
Narmeen Shammami
Research Project Manager
Brando Sordoni
Senior Associate, Accounting & Distribution
Rafi Stephan
Senior Administrative Support
Daria Tabota Associate, Marketing & Communications
Joi Tisdale
Project Manager
Jennifer Wieworka Director, Support Groups
Sandy Wilkes
Grants Manager
Yara William Associate Director, Support Groups
imfteam.myeloma.org

International Myeloma Foundation
4400 Coldwater Canyon Avenue, Suite 300
Studio City, CA 91604 USA
myeloma.org
800.452.CURE
Change Service Requested
myeloma.org

