

IMMpress Magazine
Magazine of the Department of Immunology, University of Toronto
Innovation in every reagent
For over two decades, our scientists have focused on creating unique products for the scientific community—empowering you with antibodies and reagents to find answers for your immunology research. We are constantly innovating to ensure you have the tools needed to make legendary discoveries.
Discover how our 35,000 reagents can make a difference in your lab:
• Fluorophore-antibody conjugates: dyes for conventional and spectral cytometry, including Spark PLUS and StarBright™ UV dyes.
• TotalSeq™: reagents that add protein detection to single-cell RNA or DNA sequencing in single-cell multiomic assays.
• LEGENDplex™: bead-based immunoassays for detection of up to 14 cytokines simultaneously.


• MojoSort™: column and handheld magnet-based magnetic separation systems for cell isolation and enrichment.
• Ultra-LEAF™ and GoInVivo™ antibodies: ideal for functional assays like blocking or stimulation assays.
• Cell-Vive™ GMP reagents: proteins, antibodies, and media for cell culturing.


THIS ISSUE’S COVER

“Bench” to “Business”. “Academia” versus “Industry”. These terms are often written sideby-side as an act of juxtaposition, with an intention to highlight differences over similarities. Yet “Bench” and “Business” are co-dependent entities, with one thriving due to the success of the other. Without the rigorous experiments conducted at the bench, ideation and subsequent commercialization of life-saving therapies would be impossible. Without the commercial and therapeutic success of scientific innovation, public interest and funding which are required for continued research would diminish. For this issue’s cover, I chose to visually represent both the dichotomy and mutuality of “Bench” and “Business”. The cover is clearly divided into two parts. There are two hands facing opposite directions – one is gloved and holding a tube while the other presents a pill in front of a cash-pile backdrop. Nevertheless, the two hands are inevitably a pair, as if they share a joining body and are simply protruding out of two sides of a small, black portal. Ultimately, I hope this abstract illustration provokes the readers’ deeper curiosity for how two seemingly contrasting fields, “Bench” and “Business”, not only coexist but synergize with one another.

Jennifer Ahn Design Director
EDITORS-IN-CHIEF
Meggie Kuypers
Manjula Kamath
DESIGN DIRECTOR
Jennifer Ahn
SOCIAL MEDIA COORDINATOR
Tianning Yu
SENIOR EDITORS
Jennifer Ahn
Zi Yan Chen
Baweleta Isho
Manjula Kamath
Meggie Kuypers
Vera Lynn
Siu Ling Tai
Christopher Ryan Tan
Boyan Tsankov
Nicolas Wilson
Tianning Yu
DESIGN ASSISTANTS
Larissa Abdallah
Zoeen Carter
Yashar Aghazadeh Habashi
Baweleta Isho
Meggie Kuypers
Angelica Lau
Rachel Lin
Alina Mehra
Ria Menon
Annie Mitchell
Mariam Parashos
Preya Patel
Victoria Sephton
Sophie Sun
Tianning Yu
WRITERS
Yasmin Anning
Zi Yan Chen
Milea DiPonzio
Yashar Aghazadeh Habashi
Baweleta Isho
Manjula Kamath
Meggie Kuypers
Vera Lynn
Alina Mehra
Ana Sofia Mendoza Viruega
Annie Mitchell
Mariam Parashos
Anthony Piro
Siu Ling Tai
Boyan Tsankov
Tianning Yu
FOUNDING EDITORS
Yuriy Baglaenko
Charles Tran
C ontributors
Copyright © 2013 IMMpress Magazine. All rights reserved. Reproduction without permission is prohibited. IMMpress Magazine is a student-run initiative. Any opinions expressed by the author(s) do not necessarily reflect the opinions, views or policies of the Department of Immunology or the University of Toronto.

Join our mentorship Join our mentorship team team
WE’RE RECRUITING MOTIVATED MENTORS AND MENTEES
THE GRADUATE PEER SUPPORT NE T WORK IS A FACULT Y OF MEDICINE INITI ATIVE TH AT AIMS TO IMPROVE WELLBEING OF GRADUATE STUDENTS.
OUR MENTORSHIP PROGRAM PAIRS TRAINED STUDENTS WITH MENTEES TO PROVIDE SUPPORT WITH CH ALLENGES TH AT STEM BEYOND THE SPHERE OF ACADEMIC AND CAREER GOALS.
SIGN UP USING THE QR CODES BELOW:
MENTOR: MENTEE:





The high cost of being one-in-a-million: Pharmaceutical industry makes billions from therapies to treat rare diseases
CAR-T Therapy: When medical breakthroughs bear steep price tags
Spinning Out: highlighting companies that bridge the translation gap from academic discovery to biotechnology innovation
From Coast to Coast: Canada’s Growing Immunology and Biotechnology Landscape
Biotech success story: where collaboration meets competition
MITACS: From Academia to Innovation
Thinking

Juan Mauricio Umaña: the path from a Master’s degree to Principal Research Associate at BlueRock Therapeutics
The Story of Ozempic
From Inbox to In Silico: The Role of Artificial Intelligence in Drug Development
Funding Canadian Biotech: How Venture Capital, Private Equity, and Public Programs Shape Our Innovation Ecosystem
Congratulations you’ve graduated… now what??
Building your curriculum vitae: what can you do now to improve your future job prospects?
Surviving as a PhD student in a shaky economy




FROM THE
CHAIR L etter
This newest IMMpress Magazine issue, “From Bench to Business”, focuses on scientific entrepreneurship, recent discoveries, and job opportunities within our field. This was a very comprehensive issue that should be required reading for anybody engaged in research. I think I’ll drop off a copy at the Rotman School of Management so they can have an inside view of what it is to do discovery research in Immunology that ultimately leads to therapies.
I was inspired by the scan across our department at burgeoning spin-offs, and the scan across Canada at burgeoning companies. But what inspired me the most was the story of Novo Nordisk. Of course, we know of this company as the maker of Ozempic, but its history is very relevant to a country like Canada. Novo Nordisk was born out of what used to be a fierce competition between two Danish companies that buried the hatchet and came together to become more than the sum of their parts. Not only did this actualize on some important therapeutics, but the philanthropic work of Novo Nordisk to fund the discovery sector has been an

important part of Denmark’s economy.
Despite our land mass, Canada is a small country like Denmark. We cannot afford to duplicate efforts, yet too often we are parochial and provincial. In light of our recent economic separation from the United States, we must grow out of our adolescence and become better at working together, putting on our “Canada Hats” as I like to say. A balanced diet of lab-directed and mission-directed science driven by shared goals and values is the culture we must strive to achieve. A bit of competition and a bit of collaboration in good measure have the potential to bake a better Canadian biotech cake.
Also in this issue are some positive vibes for learners wondering what is next in their careers. The Department of Immunology prides itself in training scientists that can use their powers of problem solving, persistence and critical thinking to achieve great things, whether at the bench or elsewhere. We have a tremendous track record of launching successful scientists into multiple sectors. And there’s no “mistakes” in one’s next step – I myself toggled be-
tween academic, industry and back to academia again. I learned a great deal in these different environments.
I sit and write this in late December 2025, at the end of a tumultuous year. I don’t know if you feel it, but I sense optimism in the air and a collective desire to do hard things that will make Canada a more prosperous and humane place to live. I hope you all begin 2026 with a sense of optimism – together we can do a great deal.

Jen Gommerman, PhD
Canada Research Chair in Tissue
Specific Immunity
Professor and Chair, Department of Immunology

“Will I be able to get a job in the future?”
This is a question that every grad student asks themselves as they move closer to the date of graduation. In the current global economy, employment prospects are looking more dire than ever before. And yet, as immunologists, we are fortunate to be a part of a fascinating, dynamic area of study in which discoveries at the bench are increasingly shaping real-world solutions, driving innovation across fields such as biotechnology, pharmaceuticals, and medical diagnostics. In this issue of IMMpress Magazine, we seek to highlight the pathways, challenges, and opportunities involved in turning fundamental science into life-changing technologies, ultimately preparing our fellow students for a life beyond academia.
As a background refresher, we begin with an overview on the life cycle of
L etter
FROM THE
EDITORS
In order of left to right: Tianning Yu (Social Media Coordinator), Manjula Kamath (Co-Editor-in-Chief), Jennifer Ahn (Design Director) & Meggie Kuypers (Co-Editor-in-Chief)
a biotech startup (pg.8). Our featured articles cover the modern-day success of Ozempic (pg.10), technological advancements in drug development and regulation (pg.12), and the different funding sources available to get a startup off the ground (pg.14).
It’s not easy bringing a drug to market, and we delve into some of the challenges of commercialization in areas such as rare disease treatment (pg.16) and CAR-T cell therapy (pg.18). We’ve invited Juan Mauricio Umaña, an M.Sc. alumnus who is now a Principal Research Associate at BlueRock Therapeutics, to share his story of professional success (pg.20), and we further explore the success of biotech companies in the greater Toronto area (pg.22), across Canada (pg.24), and beyond (pg.26). It may be daunting to step into the private sector for the first time, so we highlight national initiatives (pg.28) and some general tips (pg.29) to help bridge students from academia to industry. And while there is value in our degrees (pg.30), there is also a lot we can do to build up our marketable skills (pg.32) to survive in this current economy (pg.34), as well as other
career avenues to investigate (pg.36). Lastly, we wrap up this issue with a book review on Doctored by Charles Piller (pg.38), a sobering reminder of the importance of our work and how bench-side discoveries can translate into altering the course of people’s lives.
While it’s impossible to guarantee a job right after graduation, at the very least we should try to graduate with no regrets. We hope this issue can help inspire our peers to move forward with confidence in each of their own professional journeys. As always, we thank the fantastic writers, editors, and designers who contributed to this issue. As a student-run publication, this magazine truly wouldn’t be possible without you.
Co-Editors-in-Chief

Meggie Kuypers


HaveThe Life Cycle of a Biotech Startup

you ever wondered how a discovery in the lab becomes a real-world therapeutic? We all know it takes a lot more than a genius idea to build a successful start-up. In the highly-regulated biotech and pharmaceuticals space, it is an expensive and time-consuming process. After all, biology can’t be rushed. Let’s look at the different stages in the life cycle of a biotech startup.
Stage 1 – Discovery
The discovery stage starts with a novel scientific observation that has the potential to translate into a therapeutic product to address unmet clinical needs. This phase typically occurs in academic laboratories and involves identifying novel drug targets, elucidating disease mechanisms, or discovering new biologicals with therapeutic potential. Success at this stage requires not only deep scientific rigor but also an understanding of the current clinical landscape and translational potential of the discov ery.Take the example of ‘Mounjaro’, a prescription drug called tirzepatide, primarily approved for managing blood sugar levels in adults with type 2 diabetes. More recently, it has also been approved as ‘Zepbound’ for its ability to promote weight-loss. These drugs are GLP-1 receptor agonists and function by increasing insulin secretion after eating, helping to move sugar into cells, slowing digestion, and promoting feelings of fullness. This leads to reduced calorie intake and weight loss.
This discovery started in the early 1980s with research on the proglucagon gene. Proglucagon is a precursor protein that the body cuts into smaller pieces to create several different hormones, including glucagon (which raises blood sugar) and GLP-1 (which lowers it).
Key Scientific Discoveries

GLP-1 Discovery at the University of Toronto (1982 - 1996)
1. Proglucagon Processing in the Gut (1983-1984)
Dr. Daniel Drucker during his postdoc in the Habener lab in 1984 demonstrated that Proglucagon is broken down into Glicentin, GLP-1 and GLP-2 in the gut. This was distinct from the pancreas, where it did not give rise to GLP-1 or GLP-2. This tissue-specific processing was revolutionary as it showed that the same gene could produce hormones with opposite effects depending on which enzymes cut it apart.
2. GLP-1 Stimulates Glucose-Dependent Insulin Secretion (1985-1987)
Further investigation showed that a shortened form of GLP-1 directly stimulated glucose-dependent insulin secretion from pancreatic beta cells. Importantly, this only occurred under conditions of elevated glucose, providing an inherent safety mechanism against unwanted drop in blood sugar.
3. The Anorexigenic Discovery (1996)
The transformative moment came in 1996 when Stephen Bloom’s group at Hammersmith Hospital in London, along with Tang-Christensen and colleagues in Denmark, independently discovered through animal studies that GLP-1 administration reduces food intake.This discovery expanded the therapeutic potential of GLP-1 from just diabetes to obesity and metabolic syndrome, conditions affecting over 2 billion people globally.

Stage 2 – Pre-clinical Development
Pre-clinical development transforms a biological target or lead compound into a clinical candidate suitable for human testing. This stage involves iterative testing and refinement of the product. Three key areas of focus for developing typical drugs are: how the drug works in the body (pharmacology), whether it’s safe (toxicology), and whether it can be produced at scale (manufacturing). The goal is to generate sufficient data with pre-clinical models, including animal models, or cells, to prove safety and efficacy of the drug. The researchers can then file applications to investigate their identified biological as a new drug, which varies from country to country. For example, in the United States, researchers would file an Investigational New Drug (IND) application with the Food and Drug Administration (FDA), which permits human clinical trials.
Most researchers can also apply for a patent in Stages 1 or 2 to safeguard their novel product from being commercialized by others. Interestingly, the GLP-1 story followed a different model. The foundational discoveries were published openly without restrictive patents on the basic biology. This allowed multiple companies (e.g., Novo Nordisk, Eli Lilly, Amgen, AstraZeneca) to develop different GLP-1-based therapeutics.

Written by Manjula Kamath
Designed by Rachel Lin
Stage 3 – Clinical Trials
Clinical trials represent the most critical and expensive phase of drug development. The FDA requires a phased approach: Phase 1 (safety in healthy volunteers or patients), Phase 2 (proof-ofconcept in patients), and Phase 3 (pivotal efficacy and safety trials) before approval. Each phase has distinct objec tives and endpoints, and given high failure rates, for many potential therapeutics this is where their story ends.

Timeline of Tirzepatide Clinical Trials:
Phase 1: First-in-Human Studies (2016-2018)
Phase 1 trials for tirzepatide were conducted in healthy volunteers and patients with type 2 diabetes leading to dose selection for Phase 2/3 and establishing speed of action, persistence in the body, safety and tolerability.
Phase 2: Proof-of-Concept (2018)
316 patients with type 2 diabetes participated in the phase 2 trial for a 26-week period conducted by Frias et al. leading to proof of concept and approval for Phase 3.
Phase 3: SURPASS Program (2018-2022) - Type 2 Diabetes
Eli Lilly conducted five global Phase 3 trials (SURPASS-1 through -5) and two regional trials in Japan, collectively enrolling over 10,000 participants, which strengthened their case for regulatory approval. This trial demonstrated statistical superiority over the leading competitor, a critical finding for market positioning.
Phase 3: SURMOUNT Program (2019-2024) – Obesity
To gain approval for weight management in non-diabetic individuals, Eli Lilly conducted the SURMOUNT program. This trial demonstrated that tirzepatide maintains and extends weight loss achieved through lifestyle modification, compared to placebo, addressing a major challenge in obesity treatment.
Stage 4 – Regulatory Approval
Regulatory approval is the gatekeeping step between clinical development and commercialization. In the United States, the Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) reviews New Drug Applications (NDAs) for small molecules and Biologics License Applications (BLAs) for biological products. The European Medicines Agency (EMA) performs analogous functions in the European Union, and Health Canada for Canadian approval. The review process is rigorous, with regulatory body reviewers independently analyzing all clinical trial data, manufacturing processes, and proposed labeling. Approximately 90% of drugs entering clinical trials never receive FDA approval!
Eli Lilly engaged in extensive pre-submission interactions with the FDA through formal meetings since 2019. After the NDA was submitted in May 2021, it took a whole year until FDA approved it in May 2022. It was later approved by the EMA in July 2022, followed by Health Canada in November 2022, Australia in December 2022, and finally in Japan in April 2023. Eli Lilly made the strategic decision to brand tirzepatide differently for obesity (Zepbound) versus diabetes (Mounjaro). This helped them differentiate indications of the drugs for marketing and allowed separate pricing strategies.

Stage 5 – Commercialization
Commercialization represents the culmination of 1015 years of development efforts. This stage involves scaling up production to make millions of doses, building a sales team to reach doctors and patients, negotiating with insurance companies to get the drug covered, and managing the product strategically to maximize its impact and value over time. For most biotechnology startups, commercialization is handled by partners or acquirers, as building commercial infrastructure requires hundreds of millions in capital and medical commerce expertise.
Launch
Strategy for Mounjaro:
Prior to FDA approval, Eli Lilly invested heavily in manufacturing capacity, spending US$2.5B+ in capacity expansion from 2020-2023. Despite this preparation, demand exceeded supply. Demand coupled with Eli Lily’s strategic pricing in different countries, patient support programs, partnerships with Medicare/Medicaid programs, and consumer marketing has yielded astounding results. From a revenue of 274M in 2022, to an estimated revenue of 13B in 2024, Eli Lily’s two tirzepatide drugs are among the fastest growing drug launches in pharmaceutical history! Tirzepatide’s journey from a Toronto laborato ry to global medicine cabinets reflects Eli Lilly’s inspiring journey from a biotech startup to a pharm giant.

DThe Story of Ozempic
rug discovery is a game of long odds: only one in ten thousand lab discoveries survives the gauntlet from benchtop to prescription pad. Stranger still is the fate of a hormone discovered in a deep-sea creature and lizard venom, now reborn in the age of social media as a global weight loss icon – something it was never intended to be. This is the story of Ozempic, written in part by Dr. Daniel Drucker, a Toronto scientist whose work helped bring the drug to life. Before we delve in, we must pay tribute to University of Toronto scientists Frederick Banking and Charles Best, who began the first few pages of this story, with a note on diabetes.
“In science, you have a one in 10,000 chance of your research going from lab to drug.”
DIABETES, THE INCRETIN EFFECT, AND GLUCAGON
Diabetes comes in two forms, both characterized by elevated blood sugar (glucose) levels but differing in etiology. Both varieties revolve around the insulin hormone, a chemical messenger secreted into the bloodstream that regulates glucose uptake by cells. In type 1 diabetes, insulin-producing pancreatic -cells are destroyed by the body’s immune system. In type 2 diabetes (T2D), the pancreas still makes insulin, but the body resists the hormone’s command to absorb glucose, forcing the pancreas to produce more and more until it, too, begins to fail.
By the 1960s, physicians faced an epidemic of patients with T2D and few tools to help them. Researchers began searching for ways to coax the pancreas into properly absorbing glucose. Whilst doing so, they stumbled upon a curious fact: when patients ingested glucose, their bodies released more insulin and lowered blood sugar levels more effectively than when the same amount of glucose was infused directly into their veins. This suggested that the gut was communicating with the pancreas to stimulate insulin secretion in response to food. Today, this is known as the incretin effect. Scientists began testing hormones to see if they could reproduce this effect. One pancreatic hormone stood out. Glucagon, long
cast as insulin’s nemesis for its role in raising blood sugar, behaved strangely. Counterintuitively, injecting glucagon triggered a spike in insulin before blood glucose levels even rose. This paradox, that a hormone famed for undoing insulin’s work might also help summon it, transformed glucagon from villain to muse. Despite stimulating insulin release, the ultimate increase in blood glucose following glucagon administration meant it was not a suitable therapy. This, however, begged the question: is there something like glucagon that triggers insulin secretion without raising blood sugars? To answer this, researchers had to first embark on a deep dive to understand glucagon.
ANGLER FISH AND THE DISCOVERY OF GLUCAGON-LIKE PEPTIDE 1
In the early 1980s, isolating pancreatic hormones from mammals remained a challenge, posing a bottleneck for investigative studies. Scientist Joel Habener turned to the abyssal anglerfish as an alternative model organism, whose distinct pancreatic structure made it easier to isolate its hormones. Upon successfully isolating glucagon from the anglerfish, Habener’s team discovered that glucagon is derived from a larger precursor protein called preproglucagon, which can be cut into peptides. When scientists later examined the mammalian version of the preproglucagon gene, they found
a peptide called glucagon-like peptide 1 (GLP-1).
At first, GLP-1 alone did not stimulate insulin release. However, a member of Habener’s team, Svetlana Mojsov, noticed that when pancreatic GLP-1 is shortened in its length, it looks more like glucagon and acts as a powerful trigger for insulin secretion in mammals. Building on this discovery, Daniel Drucker showed that the shortened version of pancreatic GLP-1 naturally exists at high levels in the small intestine. This observation by Drucker cemented the role of shortened GLP-1 as an incretin hormone. Importantly, GLP1 only worked when blood glucose levels were high, reducing the risk of hypoglycemia – a condition characterized dangerously low blood sugar levels which is a common complication of other diabetes medication. This made the peptide ideal for patients with type 2 diabetes. Intravenous infusions of GLP-1confirmed its effect in humans, but its half-life – the amount of time it takes for the body to eliminate half of the original dose – was only about two minutes, too short to be useful as a drug.
THE GILA MONSTER AND EXENDIN-4
A potential solution was to find an alternative that was stable enough to stay longer in the body. Endocrinologist John Eng turned to an unlikely source: the venom of the Gila monster, a desert-dwelling lizard whose bite was known to overstimulate the pancreas. From this reptile, Eng isolated a peptide called exendin-4, which bore striking resemblance to shortened form of GLP-1. When administered to dogs, exendin-4 boosted insulin secretion and normalized blood glucose levels. Importantly, exendin-4 stayed in the bloodstream for hours instead of minutes, making it a viable drug candidate.
PHARMA AND SERENDIPITY
Following his successful experiments, Eng filed a patent for exendin-4 and licensed it to Amylin Pharmaceuticals, which launched clinical trials in patients with type 2 diabetes. These trials showed that patients treated with exendin-4 had significantly lowered blood glucose levels compared to those on placebo controls. Building on this success, a research team led by Lotte Bjerre Knudsen at Novo Nordisk experimented with modifying the structure of GLP-1. This modification increased the peptide’s stability and longevity, making once-daily injections of this drug feasible. At the highest doses of this modified GLP-1, patients lost around 3% of their body weight, a serendipitous discovery prompting efforts to
further optimize the molecule. Through a series of tweaks, scientists eventually created semaglutide, a next-generation GLP-1 that strikingly led to 15-20% weight loss in patients during clinical trials. This breakthrough compound is now known by its globally recognized trade name: Ozempic.
THE WORLD OF OZEMPIC
Few drugs have leapt from medical journals to late-night television quite like Ozempic. What began as a treatment for T2D has become a global phenomenon: hailed, debated, and meme-ified in equal measure. Demand outpaced supply as people sought prescriptions for cosmetic weight loss, sparking shortages for diabetic patients and ethical debates about who these drugs are truly for. Physicians were left balancing excitement with caution, reminding the public that while GLP-1 variants are powerful tools, they are not magic. They work best when treating disease, not vanity.
WHAT’S NEXT?
Meanwhile, science continues to evolve. Variations of GLP-1 have shown promise far beyond treating diabetes and obesity, with studies suggesting therapeutic benefits for heart failure and chronic kidney disease. Many of these insights are rooted in the work from Dr. Daniel Drucker’s group at the University of Toronto, whose research continues to illuminate how these hormones act across multiple organ systems. Today, his lab is exploring how GLP-1–based therapies might modulate substance use disorders and neurodegenerative diseases, which could once again expand the boundaries of what this remarkable class of molecules can do.
From angler fish to lizard venom to once-weekly injection that reshaped modern medicine, the story of Ozempic is as unlikely as it is profound. A century after Banting and Best discovered insulin in Toronto, another hormone has emerged from this city’s labs and is redefining how we think about metabolism, appetite, and chronic disease. What began as a search to help people with diabetes now sits at the intersection of biology, business, and culture. This serves as a reminder that scientific discovery often takes the most unexpected paths.
& Designed by
Yashar Aghazadeh Habashi Written

> From Inbox to In Silico: > The Role of Artificial Intelligence in
Drug Development
Ask anything
Monday morning begins: you open Outlook, fix a sentence in Word, skim a new paper, and accidentally drift onto social media where yet another “personal assistant” pops up. The recent surge of generative and natural language processing models has created the sense that artificial intelligence (AI) is capable of addressing every problem we face. Naturally, this assumption extends to drug discovery and development, a scientific endeavor long regarded as a slow, expensive, and uncertain process prone to failure. However, AI’s relationship with drug discovery predates today’s chatbots. Proteinstructure and ligand-binding predicting models established the groundwork on which modern deep learning now builds upon. From identifying novel drug targets and designing potential therapeutic molecules to predicting clinical trial outcomes, recent advances in AI offer a new paradigm for pharmaceutical research by accelerating and improving the efficiency of key processes involved in drug development.
Rather than listing AI’s strengths and pitfalls in drug development, the most meaningful way to understand its role in this multi-stage scientific process is to examine its application throughout the drug discovery pipeline. Highlighting its strengths, multiple biotech companies and startups are implementing AI to rethink long-standing workflows from hypothesis generation, candidate development, to commercialization stages.

Hello! What can I do for you today?
Identify a target, hit or lead?
Artificial intelligence in target identification and drug validation
Despite the rapid progress in understanding thousands of diseases, choosing the correct drug target can be a never-ending task. Human biology is deeply interconnected, meaning that a modification in a single element of a pathway can trigger unintended and undesirable effects. Even when a gene or protein appears promising, researchers must assess whether it can be modulated safely and effectively using reductive experimental setups that have a limited representation of human physiology. AI addresses such challenges in two major ways. Initially, it screens massive amounts of biological data that would otherwise take a long time to process. This allows the identification of relevant pathways and molecules contributing to disease and suggests potential gene-protein-drug relationships of clinical interest with high accuracy and speed. Innovations such as AlphaFold accelerate specialized drug design by providing high fidelity 3D protein structures and potential drug interaction binding models, which helps the development of companies focusing on specific areas. Genialis, for example, analyzes gene data to map tumor biology and elucidate cues that reveal key features of the cancer (biomarkers), as well as predict patient responses. Likewise, the biotech startup Ternary Therapeutics focuses on immunological disorders developing drugs that act like a “molecular glue”. Instead of blocking a protein’s activity, these drugs force two proteins to stick together, triggering the cell’s natural machinery to destroy the target protein associated with the disease.
Personal Assistant 1
Once a target is identified, the next challenge is to uncover drug candidates with promising biological or chemical activity: hits. Traditional brute-force and high-throughput screening approaches are increasingly evolving into virtual screening, where millions of compounds are evaluated in silico to predict how well they will attach to the target molecule (binding affinity) and not to unwanted ones (selectivity), whether they will cause harm (toxicity), and how practical they are to manufacture. A leading example is Numerion Labs, formerly Atomwise, founded by University of Toronto (U of T) alumni Dr. Heifets, Dr. Wallach, and Dr. Levy. This U of T Entrepeneurship startup developed AtomNet, the first deep neural network driven molecular screening platform, which transformed the paradigm of hit identification by predicting a compound’s biological effects from its structure. This approach has generated hits for antiviral targets, including Ebola, neurogenerative pathways, and dampening the immune system’s inflammatory signals, several of which have advanced into preclinical development stages. Following a similar approach, Insilico Medicine produced one of the first AI- discovered and -designed small-molecule drug for fibrosis to enter Phase 1. Achieving this milestone in only two and a half years underscores the radical time-to-market optimization facilitated by AI.
Notable AI tools are continuously evolving to decrease the high failure rate of promising drug candidates by re-evaluating hits and guiding iterative designs to maximize therapeutic potential. Canadian companies have become emerging leaders in biologic optimization. Based in Vancouver, AbCellera exploits information naturally encoded in the immune system by using large-scale AI to evaluate antibodies naturally produced against infection at a single cell resolution and selecting those with optimal properties for a particular disease or condition. Their ability to integrate tailored protein-prediction tools of transmembrane proteins, which span the cell membrane and act as communicators with the environment, has been key for their success as these proteins are notoriously difficult to study. Multiple antibodies have already advanced into Phase 1 clinical trials in fields such as endocrinology, inflammation, and soon autoimmunity. Together, these efforts emphasize that AI-driven discovery is not done by a solitary omnipotent entity. Proper guidance of human curiosity and intuition endorses a powerful partnership capable of transforming nature’s molecular repertoire into clinically meaningful therapeutics.
In conclusion, AI is reforming every stage of drug development. The discourse of AI as a replacement of human expertise must shift into a collaborative tool, where researchers must nurture tailored computational fluency to guide these models responsibly. Human curiosity alongside computational power will accelerate the delivery of meaningful therapies to patients in a time sensitive manner.
Do you need help transitioning this drug to market?
Artificial intelligence in clinical research and commercialization
AI’s influence is not limited to research and development, it also has the potential to reduce bottlenecks present across preclinical, clinical, and even commercial stages. The predictive modelling behaviour is a major advantage in the clinical landscape. Despite displaying favorable biological activity, most candidate drugs often fail because they lack the necessary properties for moving through the body and being safely cleared. AI simulations help filtering out compounds that are unlikely to succeed in vivo prior to animal testing. Additional models exploring imaging and multimodal data can detect subtle patterns and flag either positive outlooks or safety concerns that often escape human evaluation. Interestingly, AI’s support in clinical trials relies on identifying biomarkers linking treatment response outcomes and refining patient recruitment. Some systems may suggest a combination of treatments based on a patients’ characteristics. While traditional experimentation must not be replaced, AI enables informed decisions that may ultimately increase trial success and identify healthcare gaps that are often breached due to the overwhelming volume of biomedical data. Pharmaceutical companies believe AI could facilitate automated marketing channels in which patients with unmet needs are rapidly identified and drug differentiation strategies developed.
Although regulatory agencies like the FDA implement AI to assist in pharmacovigilance, by processing large volumes of reports, no AI-developed drug has received FDA approval. Major criticism relies on the quality of the data in which the models were trained on: “you are what you eat”. Since AI performance will be inherently reliant on the data characteristics, any biases and unrepresentative cohorts, among others, may distort predictions without being acknowledged. More importantly, many deep and machine learning models act as “black boxes”, where accurate outputs are generated without providing the mechanistic reasoning behind the scenes. The inability to trace testable motivations makes regulatory evaluation difficult and limits scientific trust.

Written by Ana Sofia Mendoza Viruega
Designed by Sophie Sun
Funding Canadian Biotech: How Venture Capital, Private Equity,
and Public Programs
Shape Our Innovation Ecosystem
1. VC vs. PE: What’s the Difference?
Every biotech company begins its journey with an idea, long before it earns a single dollar. To survive those early years, it needs quite a lot of capital and relies on outside investors. Two groups provide most of that financial support: venture capital and private equity. Although they are often mentioned together, they play very different roles in the life of a biotech company.
Venture capital focuses on the earliest and riskiest stages. These investors back young teams that are still generating preliminary data, refining their science, or entering early clinical work. The companies may have no revenue at all, yet strong technology and good biology make them worth the risk. Private equity enters much later, once a company has real products, revenue, and manufacturing capacity. Their money helps companies scale production, expand into new markets, or prepare for an initial public offering (IPO). , which is the first time a private company sells shares of its stock to the general public, transitioning from private to public ownership and listing on a stock exchange like Nasdaq, primarily to raise capital for growth, pay off debt, or provide an exit for

early investors. Venture capital sparks discovery. Private equity accelerates commercial growth. Understanding the difference is important, because Canada shines in early research, while it needs more strength in later stage capital and infrastructure.
2. The Canadian Funding Landscape: A Sector
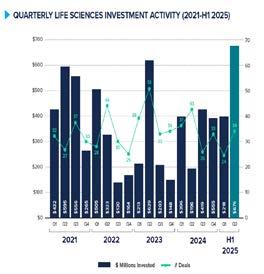
Source: Canadian Venture Capital and Private Equity Association
Growing
Despite the
Headwinds
Global financial conditions have made investment tougher in recent years, yet the Canadian life sciences sector continues to stand out. According to the Canadian Venture Capital and Private Equity Association, life sciences companies raised close to 894 million dollars in the first half of 2025 across only 58 deals. Even as overall venture activity in Canada slowed, biotech deals became larger, reflecting growing confidence in the quality of Canadian science.
This builds on strong performance in 2024, when the sector raised 1.38 billion dollars across 128 deals, surpassing the previous year despite widespread uncertainty. Investors appear willing to commit larger amounts of capital, especially to companies with compelling preclinical or clinical data.

However, the ecosystem faces important challenges. Seed and pre-seed funding, which supports the earliest academic discoveries, has been declining. Founders frequently report that laboratory space is difficult to secure, and limited access to research facilities slows the generation of the early data needed for investment. Many companies also feel pressure to move to the United States to secure larger follow-on rounds or specialized resources. Although Canada excels in basic science, gaps in early infrastructure and later stage capital make the overall path to commercialization difficult.
Even so, global firms continue to take notice. Investors from Versant Ventures and others have publicly stated that some of their most successful companies come from Canada. The science is here. The innovation is here. The question is whether Canada can build an environment strong enough to support these innovations through every stage of their growth.
3. Case Study: Lumira Ventures, Building Companies and an Ecosystem
Lumira Ventures demonstrates what a successful Canadian life sciences investment firm can look like. With offices in Toronto, Montreal, Vancouver, and Boston, Lumira invests in companies at different points along the biotech journey, from early academic spinouts to ventures approaching commercial launch.
Over more than twenty years, Lumira has funded more than one hundred companies and supported over fifty approved health products. Its portfolio companies have raised more than six billion US dollars in financing and generated more than seventy billion dollars in total revenue. These include well known Canadian successes such as Aurinia Pharmaceuticals in Edmonton and Zymeworks in Vancouver, as well as major exits such as OpSens Medical in Quebec City. Each example shows how Lumira helps transform Canadian science into real therapeutic and commercial impact.
A key strength of Lumira is its multistage investment approach. Instead of focusing only on seed stage companies or only on mature ventures, it supports companies across the full development pathway. This helps founders avoid the common funding gaps that can stall progress at critical points. Lumira also invests in talent through its Venture Innovation Program (VIP), which gives PhD, MBA, and MD trainees direct experience with scientific due diligence and investment decision making. Programs like this help grow the next generation of scientifically trained venture professionals, a resource Canada needs in order to sustain a competitive biotech sector.
4. Government Funding Programs, Incubators and Accelerators
Government programs are central to Canada’s innovation strategy. Early-stage science is expensive and risky, and public support could help companies survive long enough to attract private investment. The Scientific Research and Experimental Development tax credit is one of the most important tools for young companies, offering financial relief for costly laboratory work. The Venture Capital Catalyst Initiative, created by Innovation, Science and Economic Development Canada, expands the amount of venture funding available by investing in funds-of-funds managers and encouraging private sector participation. A dedicated life sciences stream has brought additional capital into the sector at a time when it is urgently needed.
Alongside these programs is a vibrant network of incubators and accelerators. MaRS Discovery District in Toronto provides advisory support, industry connections, and access to investors. Accelerators such as Techstars, the University of Toronto Entrepreneurship Hatchery, and the national Lab2Market program offer structured training and mentorship. Lab2Market is especially valuable for academic teams, helping researchers assess whether their discoveries can become real companies.
5. Conclusion
Canada’s biotechnology ecosystem is full of talent, innovative ideas, and scientific excellence. Venture capital supports discovery. Private equity enables growth. Government programs help bridge the gap between academic research and commercial readiness. Incubators and accelerators provide founders with space, community, and expert guidance. Yet the system still faces pressure, particularly in early-stage funding, laboratory infrastructure, and competition from larger markets. Strengthening every part of the pipeline is crucial in helping Canada turn its world class science into long-lasting real-world impact.
Written & Designed by
Tianning Yu
THE HIGH COST OF BEING ONE-IN-A

M I L L I O N
Pharmaceutical industry makes billions from therapies to treat rare diseases
Apatient living with a rare disease – defined as one that affects 1 in every 2,000 people – may understandably feel isolated. The number of patients that share their symptoms and struggles is inherently small. However, with around 7,000 different rare diseases identified to date, the rare disease community as a collective is surprisingly quite large.
Dr. Gregory Costain, a medical geneticist and rare disease expert at SickKids, said that “rare diseases are collectively common.” He elaborated that “when we say rare, people immediately think it’s something that wouldn’t apply to them or their family members or their community, [but] a significant fraction of the Canadian population is impacted directly or indirectly by a rare disease.”
Indeed, the Canadian government estimates that 1 in 12 Canadians live with a rare disease.1 In the past few years, the government has launched multiple funding initiatives for rare disease research, indicating a growing awareness of this patient population at the federal level.
For example, Health Canada announced the National Strategy for Drugs for Rare Diseases in 2023, promising $1.4 billion over three years to streamline the research and treatment of rare diseases. The CIHR also began a Rare Disease Research Initiative in 2024 to award grants to researchers including Dr. Costain, whose ongoing clinical trial evaluates genome sequencing as a potential tool for diagnosing rare diseases.
Costain is energized by these recent initiatives, and believes Canada fosters a productive environment for rare disease research. “Our public health care system […] gives [researchers] many advantages when we’re thinking about partnering with industry for clinical trials and for ultimately having drugs approved by our regulatory bodies,” Costain said.
In Canada, there are a few key agencies that help bring a drug to the market. First, Health Canada assesses the safety and efficacy of the drug. Next, groups like the Pan-Canadian Pharmaceutical Alliance settle on the drug cost. Finally, the Patented Medicine Prices Review Board (PMPRB) monitors the drug cost to ensure that it is not excessive. Despite regulatory precautions, most rare disease therapies remain extremely expensive. Remarking on rare disease
drug costs, PMPRB Executive Director Douglas Clark told the House of Commons in 2019 that “the best drug in the world won’t bring value to society if no one can afford it.”
One such unaffordable drug is the infamous Soliris, an antibody-based drug that treats the rare and life-threatening blood disease paroxysmal nocturnal hemoglobinuria (PNH). PNH causes extreme fatigue, anemia, and blood clots and affects only 6 in every million people a year. Back in 2009, the Canadian government approved Soliris as a PNH treatment, but this drug costs a staggering $700,000 per year.
Alexion, the pharmaceutical company that developed Soliris, faced widespread criticism for the excessive price of their drug. The regulatory agency in New Zealand refused to cover the cost of Soliris in 2013, since the price was “out of line with other comparable innovative new medicines supplied by other companies.”
After an extensive investigation in Canada, the PMPRB capped the price of Soliris and ordered Alexion to refund $11.6 million to the Canadian government in 2022. The company had generated $6 billion in revenue from Soliris sales in just 8 years since the drug’s introduction.
The Soliris case demonstrates that the rare disease research industry is highly lucrative, despite needing to market toward a small patient population. Fewer than 10% of all rare diseases have any available treatment, so each new drug treating a rare disease will likely have a monopoly. Indeed, Soliris is the only drug that can treat PNH, allowing Alexion to avoid competitive pricing.
Another company that has monopolized a specific rare disease treatment is PTC Therapeutics. In 2024, the company received FDA approval for Kebilidi, a gene therapy to treat an ultra-rare disease, aromatic l-amino acid decarboxylase (AADC) deficiency. AADC deficiency results from a genetic mu-

tation that prevents the production of serotonin and dopamine, chemicals in the brain essential for controlling critical bodily functions such as breathing and moving. This disease currently affects fewer than 125 people worldwide, manifesting usually during the patient’s first year of life, through symptoms like seizures and an inability to hold up their head. As the only cure available for AADC deficiency, Kebilidi clearly dominates the market, costing over $3 million per treatment in the U.S.
ment of more common neurological diseases.
Costain points to Kebilidi’s success as an example of how rare disease research can advance common disease research. “Because that disease [AADC deficiency] involves a specific neurotransmitter pathway in the brain, it’s now giving us the opportunity to explore re-applying or repurposing that gene therapy to treat Parkinson’s,” Costain said. “Again and again, we are seeing these examples of rare diseases allowing us to make the large problem more tractable.”

Though expensive drugs like Soliris and Kebilidi demonstrate the danger of monopolies in the rare disease industry, they also represent considerable scientific breakthroughs. As a gene-modifying treatment, Kebilidi delivers a functional copy of the AADC gene to the patient’s brain and drastically improves motor control. It is the first gene therapy approved to be injected directly into the brain, setting an example for treat-
The scientific insights that rare disease research can uncover may outweigh the risk of burdening health care systems with expensive drugs. “I don’t think we will get all the insights we need to get in the more common diseases from studying rare diseases, but in our opinion, that’s been one economic and scientific justification for the investment that we are making,” Costain said.
Written by Annie Mitchell
Designed by Larissa Abdallah

CAR-T Therapy: When medical breakthroughs bear steep price tags
In 2010, five-year-old Emily Whitehead was diagnosed with acute lymphoblastic leukemia (ALL), a type of cancer that affects white blood cells. After years of intense chemotherapy, bone marrow transplants, and repeated relapses that seemed to defeat all hopes for remission, Emily’s parents brought her to the Children’s Hospital of Philadelphia in a final effort to find a successful treatment. There, a clinical trial was underway to test chimeric antigen receptor (CAR)-T therapy, a novel cancer treatment that was largely unheard of at the time. In 2012, Emily became the first pediatric patient to receive this therapy – and thirteen years later, she remains cancer-free and lives a normal teenage life.

Emily’s recovery sparked global interest in CAR-T therapy and demonstrated its potential to treat otherwise incurable cancers. Since then, thousands have benefitted from this treatment. As of 2019, five CAR-T therapies have been authorized in Canada for the treatment of various blood cancers, including certain types of leukemia, lymphoma, and multiple myeloma.
However, implementation of CAR-T therapy remains challenging from logistical and financial perspectives, especially when considering, a single infusion for a patient can cost roughly $400,000 to $500,000 CAD. This article explores the science behind CAR-T therapy, the extensive manufacturing process that contributes to its high cost, and potential strategies that may render CAR-T therapy more accessible and affordable for all.


What are CAR-T cells and how are they made?


As its name suggests, CAR-T therapy is based on a type of immune cell known as the T cell. These cells play an important role in detecting abnormal or unhealthy cells, including cancer cells, in our body. CAR-T therapy harnesses this natural function of T cells but provides them with an extra “boost,” allowing them to become more potent and effective cancer cell killers.
The development of CAR-T therapy begins with isolating T cells from the blood of a cancer patient: a process called apheresis. These cells are then genetically engineered to express chimeric antigen

receptors (CARs): synthetic proteins located on the surface of T cells that enable them to specifically recognize cancer cells. The modified cells are further expanded in the lab to produce millions of CAR-T cells, which are reinfused back into the patient. Once infused, CAR-T cells can circulate throughout the body and attack the tumour cells present. CAR-T cells can also persist for years after the initial infusions, remaining armed for attack if the cancer attempts to re-emerge.




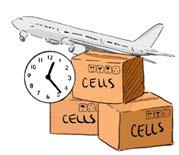
Challenges of current manufacturing practices
CAR-T therapy is unique because it must be manufactured on a patient-specific basis. Each patient’s T cells must be individually harvested and modified in specialized facilities, which requires highly skilled labor and quality control to ensure safe manufacturing processes. In Ontario, four healthcare centers currently offer CAR-T treatment. Once apheresis is completed at one of these centers, the cells are shipped to the United States for CAR manufacturing– a process that can take weeks to months before the final CAR-T product is ready to be infused back into the patient. Following the treatment, patients must be closely monitored for serious side effects such cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (iCANS). These complications can occur when the




immune system goes into overdrive, causing the release of an overload of inflammatory proteins called cytokines. CRS may present symptoms including fever, fatigue, and shortness of breath. iCANS occurs when these cytokines cross the blood–brain barrier and enter the cerebrospinal fluid, causing neurologic symptoms such as tremors, confusion, and in severe cases, seizures. Both CRS and iCANS can be fatal if not managed appropriately.
Altogether, not only is CAR-T cell manufacturing time-intensive, expensive, and resource-heavy, but the frequent follow-up appointments required to monitor these side effects further add to the overall burden on both patients and the healthcare system.
The path forward
As CAR-T therapy gains traction in the oncology field, the next challenge is expanding its reach and accessibility. Currently, CAR-T manufacturing follows a centralized model, in which pharmaceutical companies operate one major production facility in North America and another in the European Union. While centralized manufacturing helps maintain consistent product quality, it increases production time, reduces customer capacity, and drives up overall costs. An alternate strategy is a decentralized, “point-of-care” model, which involves producing CAR-T cells at or near the location where patients receive treatment. Once established, this approach could dramatically reduce wait times, simplify logistics, and lower expenses. In Canada, the Canadian-Led Immunotherapies in Cancer program is actively investigating ways to build a domestic manufacturing capacity, paving the way for “made-in-Canada” CAR-T therapies that are both faster and more affordable.

Developing “off-the-shelf” cell therapies may be another viable solution. This involves engineering T cells isolated from a single donor, using them to treat multiple patients at a time. Traditional CAR-T therapy is currently limited by the need to use the patient’s own T cells, which is essential to avoid the dangerous immune reactions that can arise from





introducing foreign donor cells. Thus, an ongoing area of research focuses on modifying CAR-T cells to make them safe for transfer between unrelated individuals. Scientists are also exploring the option of developing CAR therapies with other immune cell types, such as Natural Killer (NK) cells and invariant Natural Killer T (iNKT) cells. Similar to T cells, NK and iNKT cells have the capacity to kill cancer cells but with lower risk of triggering adverse effects. If successful, offthe-shelf approaches could transform CAR-T therapy from a highly individualized treatment into a scalable, ready-to-use product that is accessible for patients around the world.
For Emily Whitehead, CAR-T therapy meant the difference between exhausted treatment options and a cancer free life. When striving to create more success stories like this , it’s important to ask ourselves not only how we can push the scientific frontier to develop novel treatments, but how we can ensure these treatments are accessible to patients across the globe.


Written by Milea DiPonzio
Designed by Zoeen Carter


Today we’re joined by Juan Mauricio Umaña, a Master’s Immunology Program and Mallevaey lab alumnus. Juan has worked at BlueRock Therapeutics for over six years, first beginning as an Associate and later growing into his current role as Principal Research Associate. His work is split between wet lab - where he leads experimental design - and a managerial role, where he drives projects from conception to execution.
Juan Maricio Umaña: the path from a Master’s Degree to Principal Research Associate at BlueRock
Therapeutics
Can you tell me about your role and what your day-to-day routine looks like?
“It’s a very collaborative environment,” Juan explains. “As a Principal RA, I help design experiments alongside my team, define what questions we want to answer, and drive the projects forward. It’s not just about doing one experiment at a time- it can be about managing an entire research process!” His schedule balances both hands-on lab work and planning. Roughly half of his week is spent conducting experiments, analyzing data, and writing experimental workflows; the other half involves meetings and discussions to guide research direction. “It’s about asking the right questions, planning the next steps, and making sure what we do fits within the bigger project timelines,” he adds.
Sounds like what you’re doing now is like what you did during your graduate degree... what kind of differences are there being in a more business-adjacent setting?
While the scientific curiosity and structure of his work feel similar to graduate school, Juan notes several key differences in the industry setting. “In grad school, you’re often the entire team,” he says, “you do everything by yourself. In industry, everything is collaborative. You lean on other people’s expertise, and you don’t have to reinvent the wheel every time.”
He also highlights the importance of structure and planning. “In grad school, you can sometimes take more time to explore different approaches. But in industry, your work is often part of a bigger puzzle. Others depend on your results. Timelines matter. If you’re developing an assay, for
example, you need to plan when it’ll be ready so that you can hand it off to the next team.”
Being in grad school can be great for picking up soft and technical skills – which ones do you find have been helpful in your career now?
Juan credits his Master’s degree for shaping both his technical and soft skills. On the technical side, he mentions experimental design, flow cytometry, and meticulous documentation. These are skills that translate directly into his current role. “In grad school, I was a bit loose about documenting experiments,” he laughs. “But in industry, that’s crucial. Everything must berecorded in detail so that anyone can replicate your work.” He also emphasizes communication and adaptability. “Grad school teaches you how to present complex data to different audiences. In industry, that becomes even more important. When I present to my manager, I might go into detail. But if I’m talking to executives, I need to focus on the key outcomes that help them make decisions.” Another major takeaway from graduate school was mentorship. “I was a TA and had undergraduates in the lab, and that really helped me learn how to teach and lead,” Juan reflects. “Now, mentoring junior associates or co-op students is a big part of what I do. It’s rewarding to help others grow while also learning from them.”
An important question for those of us graduating soon… how did you find your position?
Juan’s path to BlueRock Therapeutics came naturally from his academic background in immunology. After finishing his MSc, he completed a six-month research position in transplant immunology at UHN before discovering the opportunity at BlueRock. “I think the stars aligned,” he recalls. “They were looking for someone with an immunology background, and I was fortunate to have the right combination of skills and experience. But I also believe in the idea that you create your own luck... you know what they say, opportunity is when luck meets preparation.”
Thank you for your time today! Any last advice for current graduate students?
Juan’s advice centers on humility, patience, and a willingness to keep learning. “Be receptive and patient,” he says. “Science is about realizing how much you don’t know. The more you learn, the more you understand how little you really know—and that’s okay.” He also stresses the importance of transparency and initiative, especially when working with supervisors or managers. “If you make a mistake, be upfront about it. That’s how trust is built. And when you bring a problem to your manager, come up with po -
tential solutions too. It shows initiative and maturity as a scientist.” Finally, he encourages graduates to stay open to growth. “Even now, I’m learning new technologies all the time. The field evolves fast, and you need to evolve with it. Stay curious and adaptable—that’s what will carry you forward.”
Juan Mauricio Umaña’s journey from MSc student to Principal Research Associate reflects how a foundation in curiosity and collaboration can lead to a thriving career in the industry. For students that wish to hear more about his experiences during his graduate studies or during his current position at BlueRock Therapeutics, Juan has consented to being contacted through his LinkedIn profile.


Written by Vera Lynn
Designed by Ria Menon
Spinning Out: highlighting companies that bridge the translation gap from academic discovery to biotechnology innovation

The field of biotechnology, or more commonly known as “biotech”, implements the use of living organisms to develop applications intended for improving human welfare. Medical biotech companies pursue or license in scientific discoveries to produce tangible benefits for healthcare such as therapeutics, diagnostics, and vaccines. In some cases, these companies emerged from a novel technology with the potential to improve human health, inspired by a discovery that has been “spun off” from an academic lab.
Laboratory trainees, like us, stand at the start of many of these breakthroughs by coming across findings that show great potential. This is the start, but it is not simple to commercialize a technology. There are numerous steps with complex legal jargon to follow once the process is started. Here, we will highlight some major components in this process: First, it’s important to determine whether commercialization is viable. The next steps that follow include building a founding team, negotiating with the technology transfer office, obtaining rights for intellectual property (IP), hiring advisors and employees, and acquiring funding. Luckily, many universities strongly support commercialization of scientific technologies with established programs to aid startups.
Start-Up Resources


At University of Toronto, we have an entrepreneurship community which consists of 12 innovation hubs called Accelerators, geared towards providing support, resources, and partnerships in various fields. University of Toronto Early-Stage Technology is an accelerator that focuses highly on partnership and funding, with ties to the surrounding hos pitals, MaRS Discovery District, and Toronto Innovation Acceleration Partners (TIAP). At UTM, SpinUp, termed a “wet lab incubator”, provides early-stage resources, such as subsidized lab and office space, to allow startups to focus on developing the IP. These programs are designed for building up entrepreneurship and bridging the translation gap from research to technology.
Biotech Metrics


When comparing these spin-off companies to the overall biotech sector, we can look at a few metrics. Approximately one-third of biotechnology firms originated as spin-offs, with the majority originating from academic research institutions. Spin-offs are typically smaller and have a greater focus on research and development compared to well-established biotechnology firms. While most spin-offs do not grow into large, public companies, the bulk of them have high survival rates at 5 years, and some even longer.
Radiant Biotherapeutics

Our very own Immunology department is home to labs that founded a major spin-off biotech, Radiant Biotherapeutics. The research conducted in the labs of Dr. Jean-Phillipe Julien and Dr. Bebhinn Treanor resulted in a patentable technology called Multabody. Multabody is an antibody platform with the “mult” in the name referring to its multi-valent and multi-specific properties. The basis behind this innovation utilizes apoferritin, a human scaffold protein that can self-multimerize, which when combined with antibody fragments can assemble into a multimeric structure. The technology is modular meaning that the antibody fragments can be swapped out depending on the target. Because of this, Multabody has a wide range of potential therapeutic targets. Radiant Biotherapeutics’ current pipeline includes targets in oncology, inflammation, and HIV. In their oncology portfolio, their lead clinical candidate is RBT-101, a 4-1BB agonist. Recently presented at the Society for Immunotherapy of Cancer 2025, it was highlighted that RBT-101 could induce tumour regression without liver toxicity in a mouse tumour model. From this, Radiant Biotherapeutics will continue to develop their lead oncology program, as well as other Multabody platforms for therapeutics in disease.
Triumvira Immunologics

UofT is not the only place where academic research fuels industry. Triumvira Immunologics is a biotechnology company founded in 2015 by Dr. Jonathan Bramson at McMaster University. Their major focus is cancer therapeutics, with their main proprietary technology being the T cell Antigen Coupler (TAC) molecule. The TAC molecule is a chimeric molecule with 3 domains: the antigen binding domain which binds to the tumor cells, the CD3 binding domain that interacts with the T cell receptor (TCR), and the CD4 Co-Receptor Domain, which is the proprietary component of the Triumvera technology. The CD4 Co-Receptor Domain is what anchors the TAC molecule into the cell membrane and is responsible for activating the immune response. The company’s leading clinical candidate is TAC101-CLDN18.2, an autologous TAC program that uses a patient’s T cells genetically engineered with a TAC molecule to redirect T cells to target tumors expressing Claudin 18.2, a protein on solid tumors. Preliminary results from a phase I/II study were presented at 2025 ASCO Gastrointestinal Cancers Symposium and Triumvira is continuing to develop this candidate as well as others to diversify the applicability of the technology in cancer treatment.
More Spin-offs...
A few other companies that have “spun-out” include Notch Therapeutics, a company using stem cell-based technology to develop cell therapies in cancer application, founded by Dr. Juan-Carlos Zúñiga-Pflücker and Dr. Peter Zandstra, also formerly from UofT. Dr. Nathan Magarvery founded Adapsyn out of McMaster University to leverage bioinformatics to discover novel molecules with potential for benefits in various therapeutic areas, agriculture, and nutrition. Perturba Therapeutics was founded by combining the discoveries from Dr. Igor Stagljar’s lab at UofT with AI-augmented chemistry technology from a biotech company called Cyclica. Finally, within the past year Lunar Therapeutics was founded out of University of British Columbia, focused on cell therapies.




Overall, “spin-off” biotechnology companies are a small portion of the biotech world, but they represent the vast potential of academic research in scientific progress. The process to commercialize a discovery is a long process but has the potential to produce great benefits in healthcare. Ultimately, Canadian academic research labs spinning out into biotechnology companies are essential in bridging discovery to translational applications.
Written
& Designed by
Alina Mehra





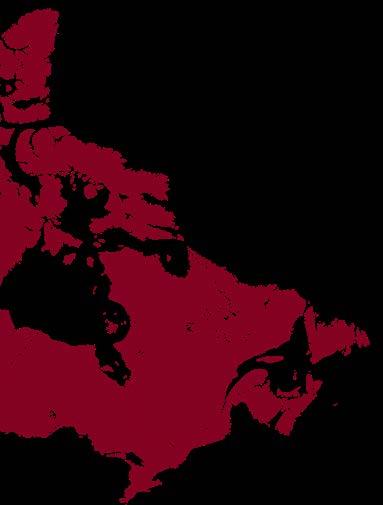
From Coast to Coast: Canada’s Growing Immunology and Biotechnology Landscape
Canada is a country defined by its sheer size, which gives rise to diverse landscapes and distinct regional cultures. From the wet and temperate mountains of British Columbia, across the open plains, to the densely populated cities of Ontario and Quebec and the rugged seaboard of the Maritimes, Canadians have established communities within every region. Although each are distinct from one another, a common thread across these communities is a shared passion for science, discovery and biotechnology. This passion takes form not only in the nation’s vast network of universities, research centres, and large-scale foreign pharmaceutical company sites, but also in the creative and innovative biotech companies founded on Canadian soil.
It takes just one dedicated individual, or team, with a single idea to harness their creativity and scientific knowledge to bring an idea into reality. As novel discoveries are made and technologies are applied in new ways, the scientific foundation for new biotech companies constantly evolves. Of all the fields guiding these innovations, immunology stands out as one that has never been more relevant in the current landscape. Across Canada, this is reflected in the rise of biotech companies translating breakthroughs in immunological science into life-saving therapies and diagnostics - from West to East.
Vancouver, BC: Zymeworks
Starting far off in the West, Vancouver is home to one of Canada’s most successful biotechnology companies, Zymeworks. Zymeworks specializes in the design, development, and production of antibody therapeutics that treat cancers, autoimmune disorders, and inflammatory diseases. Zymeworks’ success lies in their ‘Azymetric’ antibody design platform, which enables the design and screening of highly customized antibodies capable of safely and effectively targeting specific tumour cells.
While this technology has led to the development of a diverse array of antibody shapes and forms, Zymeworks has achieved particular success with ‘bispecific’ antibodies which target a given protein in two distinct regions for increased specificity. This approach led to the development of zanidatamab, an antibody targeting the HER2 protein, commonly overexpressed in many metastatic breast cancers, which received accelerated approval in 2024.
Beyond zanidatamab, Zymeworks also specializes in the design and development of antibody drug conjugates, which involves binding antibodies to drugs for increased delivery specificity. By developing novel targeting and conjugation techniques, Zymeworks has effectively created a versatile “toolbox”


that allows scientists to mix and match antibody specificities with different drugs, leading to several new therapeutic candidates. With multiple drugs in clinical and preclinical development, and ongoing collaborations across the pharmaceutical industry, Zymeworks stands as a beacon of Canadian biotechnology driving global innovation.
Toronto, ON: Noa

Next, a prime example of Canadian innovation exists directly across the street from the University of Toronto campus. The MaRS Discovery District is North America’s largest urban innovation hub and home to many medical, engineering, and information technology start-ups. Among them, Noa Therapeutics exemplifies how Canadian researchers are tackling chronic, immunological diseases from new angles. Their mission is to develop long-term and effective treatments for chronic inflammatory diseases by harnessing the power of the aryl hydrocarbon receptor (AhR), a protein involved in the regulation of immunity, metabolism, and barrier function genes. Inflammatory diseases are hallmarked by the dysregulation of these systems, and as such, through
fine-tuning of AhR activity by designing and developing targeting-drugs, the company aims to restore immune system and barrier balance.
Noa has developed a discovery engine to screen for safe and effective AhR targeting compounds, which has led to their current lead candidate, NOA-104, an atopic dermatitis therapeutic currently in pre-clinical testing. While still early in development, Noa’s work is already generating buzz; this is evidenced by the Catalyst Research Grant recently awarded by the National Eczema Association to Johns Hopkins’ University Department of Dermatology to fund in vivo studies of the compound.
As one of many promising ventures within MaRS, Noa highlights the opportunity for creative, early-stage experimentation in Canadian biotech sphere and exemplifies how Canadian
innovators are pushing the boundaries of translational science from the lab bench to the real-world.




A common use of computational methods is in early-stage discovery and design, and is a key component of Montreal-based Cura Therapeutics’ discovery platform. Cura Therapeutics is working to develop next generation cancer and age-associated disease immunotherapies through the design of single drug compounds capable of targeting disease from multiple angles. Notably, their platform integrates AI-driven predictive protein modeling with immune functional assays to bioengineer and optimize these therapeutic molecules. Their leading candidate, CT101, is shown to have immunostimulatory, anti-metastatic, and anti-angiogenic properties, making it potentially the first of its kind if approved. Cura is now expanding its AI-driven discovery platform to design drugs for additional diseases, showcasing how Montreal’s growing computational science ecosystem is accelerating the creation of innovative therapies that address diverse and complex diseases effectively.


Halifax, NS: MedMira
Finally, although often considered more remote and secluded, the Maritimes offer unique advantages for establishing biotech companies that represent Canada on the global stage. A lead example is MedMira, a company developing the next generation of rapid diagnostics for sexually transmitted and emerging diseases, including COVID-19.
Built upon the immunological antigen-antibody interaction, MedMira’s products uniquely detect multiple biomarkers of a given disease on a single test, allowing for highly specific, rapid, and accurate testing. MedMira was founded by graduates of Acadia University in Wolfville, Nova Scotia in
1993, and has remained headquartered in the province since. Its co-founder cites a steady stream of talented local graduates and proximity to both the U.S. and Europe markets as major advantages of their location. MedMira stands as a testament to how innovation and global impact can thrive even outside major urban centers, highlighting the depth and reach of Canada’s biotechnology ecosystem.


Moving slightly east and just a short train ride away to Montreal, another innovative technology hub is rapidly expanding. What’s unique about Montreal, however, is its emergence as Canada’s artificial intelligence and machine-learning centre. Given this, computational expertise is increasingly being integrated into biotechnology development in the city, including in emerging immunotherapies.
From Vancouver to Halifax, Canada’s biotechnology landscape reflects a nationwide spirit of innovation grounded in scientific excellence. Whether through antibody engineering, immunomodulatory drug discovery, AI-driven therapeutics, or rapid diagnostics, Canadian biotech continues to push boundaries and transform immunological research into real-world medical solutions.
Written by Anthony Piro
Designed by Victoria Sephton
When we observe the previous winners of the Nobel Prize, it becomes immediately apparent that open collaboration, and a drive to improve humanity’s fight against disease are important factors of conducting impactful science. Sharing her views on the future of CRISPR and gene editing, Dr. Jennifer Doudna states: “The research that I’ll talk about today wouldn’t have happened … if I had been working anywhere else. And that’s because we have a really collaborative environment on our campus”. Her collaborative approach to science is further reflected in her joint work with Dr. Emanuelle Charpentier for the engineering of the CRISPR-Cas9 gene editing system which won them the Nobel Prize in 2020. On the other hand, when thinking about establishing a successful company, founders and entrepreneurs alike typically think of ways to beat the surrounding competition. Unlike the language used to describe scientific discoveries, war-like terms are often incorporated in business-speak such as “disruptive”, “competitive”, and “strategic”. Contrasting perceptions of what drives successful science versus a profitable business might lead one to believe that a scientific mindset is incompatible with strong business acumen. In some cases, this may be true. However, the founding of Novo Nordisk – currently the world’s largest biotechnology company – shows us that competition and collaboration can paradoxically work together to foster business growth and propel the healthcare of humanity forward.
Following his Nobel Prize win for discovering the mechanism of blood perfusion through capillaries, the Danish scientist Dr. August Krogh went to give lectures at universities on the American east coast. Persuaded by his wife who had been struggling with type 2 diabetes, he made a special stop at the University of Toronto to meet Frederick Ban-
ting, Charles Best, and John Macleod – scientists who had successfully isolated insulin that could be used to treat diabetes. Motivated by his wife’s condition, Krogh managed to receive their permission to manufacture active insulin in Europe. Then in 1923, he partnered with a diabetes expert, Dr. H.C Hagedorn, and a pharmacist, August Kongsted to establish the Nordisk Insulinlaboratorium in Copenhagen. The goal of this company was humanitarian in nature: firstly, to produce insulin at scale to help patients with diabetes, and secondly to advance science through the creation of grants funded by the company’s profits.
As Nordisk Insulinlaboratorium expanded, internal conflicts began to occur. In 1924, Hagedorn fired a valued chemist at the company, Thorvald Pedersen, due to a disagreement, which also led to the resignation of his brother, Harald Pedersen. The Pederson brothers wanted to continue the Nordisk Insulinlaboratorium’s mission of making insulin and decided to compete with the incumbent. August Krogh famously told Harald “you’ll never manage [to make insulin]” – words that would inevitably come to back to bite him.
The Pedersen brothers established their new biotech company, Novo Laboratory, merely a few kilometers away from the Nordisk Insulinlaboratorium. Whereas Krogh, Hagedorn, and Kongsted used their status as social and intellectual elites to grow their operation, the Pedersen brothers made up for their less impressive professional network and credentials by boasting a strong entrepreneurial spirit, business acumen, and perseverance. The brothers’ grit and dedication to the financial survival of the Novo Laboratory proved to be an effective answer to its well-connected and

methodical rival. Novo Laboratory eventually transitioned to becoming the Novo Foundation, which became lucrative and in turn awarded grants to life sciences research. The competition between Novo Foundation and Nordisk Insulinlaboratorium in the 1980s was so fierce that they were not only competing within the same market, but also for the same scientific personnel and researchers within Denmark.
An event in 1989 proved that business sense and scientific collaboration can work together to create a product that is larger than the sum of their parts: the merger of the Novo and Nordisk Laboratories into the widely-known company Novo Nordisk. Fueled by the desire to create a global business leader in the pharmaceutical space, and the wider humanitarian goal to create a unified foundation that would provide grants for life sciences research, the merger was one of the largest in Denmark’s history. The financials have indeed worked out in both companies’ favor. In 2023, Novo Nordisk accounted for an impressive 8.3 percent of Denmark’s entire gross domestic product, and in 2024, their market cap was an immense 445 billion USD. In fact, the contribution of Novo Nordisk to the Danish economy is so large that the entire country is impacted during recessions in the biotechnology sector. Nevertheless, the Novo Nordisk foundation has also had massive philanthropic success since the merger. The 1.4 billion USD awarded by the foundation
in 2024 helped launch Denmark’s first supercomputer as well as fund academic research on stem cell and regenerative therapies. It is undeniable that the company’s humanitarian investment in life science research will lead to an improvement in human health.
Collaboration epitomizes some of the best parts of the human spirit – a desire to gather our collective resources and knowledge to create a product that is greater than that which we would have made individually. However, despite its sometimes-negative connotation, competition also has a vital role in humanity’s greatness. It can instruct us to put our best foot forward and strive for perfection when faced against competitors. Therefore, if individuals manage to synthesize and bridge the gap between competitor and collaborator, humanity ultimately benefits, as shown by the story of Novo Nordisk.
Written by Boyan Tsankov
Designed by Meggie Kuypers

MITACS: FROM ACADEMIA TO INNOVATION
For many students and post-doctoral fellows, the leap from the lab to the world beyond academia can feel uncertain. How do you take your research skills and apply them to solve real-world challenges? Closing this divide is Mitacs, a national non-profit organization that connects Canadian post-secondary institutions with industry. At the nexus of academia, government, and public and private sectors, Mitacs is Canada’s leading innovation organization that works to mobilize the brightest minds through internships and professional training to solve multidisciplinary societal challenges.
Founded in 1999, Mitacs began with a focus on mathematics, but has since expanded into nearly every discipline from STEM to social innovation. At its core, Mitacs operates through collaboration. With 13,000+ academic supervisors, 35,000+ innovation projects, and over $1.42 billion invested in research and development, it finds local and international talent to turn ideas into solutions. Whether it’s advancing digital technologies, renewable energy, artificial intelligence, or sustainable health innovations, Mitacs helps translate academic research into tangible outcomes that benefit both science and society.
For Canadian post-secondary students, post-doctoral fellows, international students, and recent graduates eager to gain experience, Mitacs offers an array of internships and funding programs tailored to different goals and stages of innovation:
• Pairs students/fellows with for-profit or non-profit organizations to conduct research projects.
• Participants receive $15,000 for a four- or six-month internship to help businesses optimize their budgets, grow their research and development, and solve research challenges.
• Provides $10,000–$15,000 for a four-month placement focused on helping Canadian companies enhance their products, processes, or services.
• Focuses on business planning, operations, finance, and marketing, dependent on the needs of the organization.

• For those looking to initiate their own start-up, Mitacs provides funding to help develop new technologies, products, or services.
• Each internship provides $15,000 to help pilot test, refine, and move promising innovations toward commercialization.
• This internship offers international undergraduate students a fully funded 12-week summer research placement in Canada.
• GRI matches students with Canadian research faculty across diverse disciplines for hands-on experience in their field of study.
• Mitacs funds the entire process, including travel, logistics, event expenses (conferences, symposia, workshops, etc.), and administrative support.
• Interns are matched with mentors affiliated with Canadian universities to ensure a smooth experience.
Across all programs, interns gain access to the Mitacs e-Campus, an online platform offering free professional development courses designed to boost employability and ensure project success. Through Mitacs training, participants are taught by industry experts in communication, leadership, management, and entrepreneurship. They offer flexible modules ranging from 60 to 180 minutes long. Upon completion, participants receive certificates that recognize their new competencies and commitment to professional growth. By nurturing both technical and interpersonal skills, Mitacs helps shape researchers who are not only scientifically skilled, but also adaptable, collaborative, and creative. For students, post-doctoral researchers, and young scientists alike, it represents an opportunity to explore industry, transforming curiosity into impact and knowledge into innovation.
Written & Designed by
Mariam Parashos
ACCELERATE PROGRAM
GLOBALINK RESEARCH INTERNSHIP
THINKING OF STARTING YOUR OWN BUSINESS?
Starting out can often feel overwhelming, but with the right mindset and preparations you can set yourself up for success. Here’s a handy checklist to guide you through the early stages of starting a business:
DO
1. Make a solid business plan
Start out with a solid idea. Map out your goals, target market, costs, and revenue streams. Your plan doesn’t need to be super long or detailed, especially at the beginning, but it will give you a framework to measure your progress. Excellent business plans can even help attract investors!
2. Know your market
Research, research, research! The more you understand your market, the more effectively you can find your place in it. This includes knowing your competitors, the current trends, price points, and what makes your business stand out from the rest. Lowballing costs and overestimating revenue are a surefire way for early business collapse.
3. Be open to adjustments
Your first idea may not be perfect, and that’s okay! Stay flexible and adapt based on feedback and market changes. Make sure you continuously monitor your progress and update your plans accordingly. Don’t let the fear of changing your ideas hold you back — after all, you know what they say about the best laid plans…
4. Ask for help
Seek advice from mentors, join entrepreneur networks, and don’t hesitate to consult experts in finance, marketing, or law. Collaboration accelerates growth and oftentimes recognizing that you need help — and being able to ask for it — is a valuable skill in and of itself.
DON’T
1. Try to do everything yourself
You’ll burn out quickly and, as much as you might not want to admit it, you’re definitely not the best person to be doing every task. Delegate responsibilities, outsource when needed, and build a strong team that you can trust to form the core of your company.
2. Rush into things
Take the time to validate your idea, build your foundation, and secure resources before launching or expanding your business. Thorough preparation can prevent costly mistakes. Don’t assume growth will always continue linearly — slow and steady will help you win any race!
3. Underestimate the effort
Running a business takes time, persistence, and patience. Expect long hours and plenty of obstacles before you even get things off the ground. Building startups can very well take over every aspect of your life, and you need to be mentally prepared before jumping in.
4. Be afraid to fail
Failure is a part of learning. Every setback teaches valuable lessons and brings you one step closer to success. Companies can fail for a number of reasons, only some of which are in your hands. Don’t be discouraged and keep a level head when things don’t pan out the way you expect.
Designed by Aniela Mitchell
Written by Meggie Kuypers

Congratulations you’ve graduated… now what?
In 2024-2025, there were 3,183 graduate learners in the Temerty Faculty of Medicine; just shy of 50% of these are enrolled in a PhD program. Upon finishing their degrees, these graduates will join fellow alumni that make up 9% of the Canadian population — a percentage that is trending to increase every year, according to the most recent 2021 Statistics Canada survey. From our Department of Immunology, a department sized around 130 students, between March-September of this year, 16 students were admitted to the Master’s degree and 7 students were admitted to PhD degree.
Yet, the majority of these graduates don’t end up pursuing careers as university professors and lecturers. According to a Statistics Canada survey conducted in 2016, approximately 26% of male graduates and 23% of female graduates from physical and life sciences end up pursing traditional academia positions (however the percentage increases approximately 2-fold in both categories if they graduated from Education, the highest discipline producing academics). So why pursue further graduate education then? One motivation could be increased salary. According to the most recent Statistics Canada data collected from 2020, the median average salary of someone with a Master’s degree is $80,000 whereas with a PhD, it is $89,000. This is compared to someone with a Bachelor’s degree, with a median salary of $64,000. Keep in mind that this number in Canadian dollars is a rough estimate and isn’t adjusted for present inflation, nor discriminates across genders, years of work experience, or line of work.
But salary is not the only indicator of graduate alumni success. Arguably, pursuing graduate studies should be a challenging, transformative experience that will leave you with strong critical thinking, analytical, and communication skills – not to mention a whole ton of grit you never thought you had.
Philosophically speaking, obtaining a graduate degree in research requires a complete reshaping - or dare I say –dismantling - of what we have been learning in school for 16+ years. For years, we have been building a foundation
of knowledge that is non-interactive in the act of learning. Sitting comfortably with rote memorization and application of theories in limited contexts (for convenient exam testing purposes) suggests that all knowledge has a binary “correct” versus “wrong” framework. When we do research, we cross into uncharted territory. We have to sit actively wrestle with unknowns and “maybes”, sometimes for weeks or years on end. While this mentality might be frustrating, one benefit of graduate studies is that we develop a useful tool set of imagination, critical thinking, and perseverance.
A second tool we acquire during graduate studies is analytical thinking. Data analysis is a critical, versatile, highly sought after skill in multiple career fields. Through our training with testing hypotheses, we need to be able to discern the truth from the noise via proper design of experimental testing conditions. Evaluating statistical significance enables us to make decisions and predict with more confidence – key skills in areas of risk management.
A third benefit of pursuing graduate school is knowing how to tell a story upon interpreting experimental findings. There are multiple examples of work where data needs to be collected, organized, and presented; however, the work is more meaningful when it connects what is already known with what remains unknown. Being able to simplify the research project’s main question and findings is a challenging feat, particularly in lay terms. Graduate students are encouraged to apply for their own scholarship funding, present their work in poster and oral talks at conferences, and write their own abstracts in addition to submitting their peer-reviewed manuscripts. Through these practices, they learn how to condense several months to years of work into an impactful “elevator pitch”. These communication skills are vital not only to help others understand the broader impact of their work to society but also to continuously remind oneself of the significance of one’s work. Students in the Department of Immunology also get to practice their scientific communication skills to lay audience members through student-run initiatives such as IMMspire, an interactive workshop teaching immunologi-
cal skills and knowledge to youth, and IMMpress. Being able to communicate clearly and precisely is significant and translatable to many fields ranging from medicine, law, business, and news reporting.
A fourth benefit of graduate school is developing our network. Many graduate students, at least once during their degree, are given an opportunity to attend local and/or international conferences. These opportunities improve on skills related to presenting research findings to people who may be unfamiliar with your research field. Throughout our graduate degree, we begin to build this confidence by discussing with others in our field and gives us the ability to bounce ideas off each other. Another subtler aspect of networking is through the accumulation of knowledge in one’s particular field, we familiarize ourselves with labs doing similar work. Potentially, these labs could become future collaborators. Opportunities such as these in graduate school don’t come often and should be seized at the earliest chance. Even if one doesn’t plan on continuing with academia in the future, there are plenty of opportunities to develop networks with fellow graduate alumni in other careers and industries.
In summary, pursuing graduate studies enables us to develop an arsenal of translatable skillsets for many careers. In medieval times, the Master’s degree was once reserved for the pursuit of specific, advanced subject matter including
arithmetic, astronomy, music, and geometry, while the PhD covered theology, medicine, and law.6 Hundreds of years later, the range of opportunities for professional development has dramatically expanded. In our own Department of Immunology, the former 2-year thesis-based Fundamental Master’s program was replaced in 2020 with the 1-2 year Applied Immunology program, that offers an attractive 4-month internship experience. The alumni of this program have now embarked on a wide range of careers including but not limited to medical writing and consulting. For PhD graduates, one post-graduation track is additional postdoctoral training to provide opportunities for more experience, leading to independent projects and networking opportunities, however PhD graduates may also consider other lines of work including industry associate scientist roles, medical liaison positions, consulting, teaching, among others. Regardless of the path(s) you choose, when you cross that stage at Convocation Hall, take the time to savor that well-deserving moment and pat yourself on the back. You’ve finished the journey, ascended the peak of the mountain, and that should be, in and of itself, fulfilling.
Written by Zi Yan Chen
Designed by Baweleta Isho

Building your curriculum vitae: what can you do now to improve your future job prospects?
Thecurriculum vitae, or CV, is a document that all academics have prepared numerous times during their careers. Latin for “course of life,” the CV is a detailed professional summary of all academic qualifications, work experience, and career-related achievements that is often used for the purposes of employment or grant applications. The CV presents a holistic overview of your career, including but not limited to education, publications, and presentations given.
While in some parts of the world the term “resume” and “CV” are used interchangeably, that is not the case in Canada and the US. CVs differ from resumes in many ways including: the length of the document (a CV often has no page limit, while a resume is short and concise on a maximum of two pages) and the career you are applying for (a CV is often used for academic or research jobs, while a resume can be used for many kinds of jobs). Care should be taken when choosing which format to use, taking into consideration where you are applying and what employers may be asking for.
In this article, we will discuss the strategies to improve your CV while you are a graduate student, which in turn will hopefully benefit your future job prospects.

What to include on your CV?
To understand how to improve your CV, we must first understand what a standard CV should include. As mentioned, your CV should chronologically detail your academic qualifications including degrees obtained, professional work experience, publications (those in preparation or fully published), and presentations given (oral
or poster). CVs can also list grants and scholarships awarded and their monetary value, in addition to professional associations, volunteering experiences, courses taken, certificates gained, students mentored, patents and trademarks, interview and media relations, and many other professional achievements. Whether these aspects are listed are highly dependent on the position or grant you are applying for, as it is important to tailor your CV to each application. For example, if you are applying for a teaching position, including a summary of students mentored within your teaching experience would be a valuable addition to demonstrate you possess the relevant experience and skills that the hiring team may be looking for. A tool that supports the building of CVs is the Canadian Common CV (CCV), which can be accessed at https:// ccv-cvc.ca/. Often used for grant applications, the CCV is a national website that helps keep track of professional achievements and can be easily adapted to include sections based on need. It is encouraged to create your CCV and keep it up to date to ensure all professional achievements are accounted for appropriately. But what can be done while we are still in graduate school to improve our CV? This is dependent on your interests and goals. There are plenty of opportunities if you know where to look.
LSCDS. The Life Sciences Career Development Syndicate is an initiative run by graduate students and postdoctoral fellows. It is officially recognized by the Temerty Faculty of Medicine and works to bridge the gap between academia and industry by raising awareness of career options available post-graduation. This initiative holds a variety of events that could be included on CVs, including but not limited to career seminars, skill-building workshops, mentorship opportunities, and industry team case study programs. The unique opportunities organized by the LSCDS are available to current graduate students, postdoctoral fellows, and research associates across the University of Toronto and affiliated teaching hospitals.
Mini-MBA. If you’re looking into a career in business, consulting or management, the Graduate Management Consulting Association and the University Health Network have collaborated to design “Business Fundamentals,” a 10-week

online course that introduces graduate students and postdoctoral fellows to various concepts in business. The course culminates with an application of the concepts learned by solving a business problem within a case study that is judged by industry professionals. At this time, the program is offered only to individuals affiliated with the UHN, however similar programming is also available through the Rotman School of Management MBA Essentials course. Completion of either program can be included on your CV.
Graduate School Internships. If industry interests you, opportunities to participate in internships with pharmaceutical companies are also an excellent opportunity to improve your CV. Pfizer Canada, for example, has an internship available in Medical Affairs. Open to university-level students studying the health sciences, this internship focuses on developing content related to Medical Affairs. Giuliano Bayer, a senior graduate student in Dr. Dana Philpott’s lab, recently completed this internship. In some cases, these internships are hybrid and can be done in conjunction with your academic degree. These kinds of opportunities are great for improving your CV while also expanding your professional network.
Teaching Certificate Programs. For those of you with an interest in teaching, the Teaching Assistants’ Training Program (TATP) is a peer-training program that provides pedagogical support through numerous certification programs for teaching assistants and graduate students at the University of Toronto. Organized by teaching assistants, the TATP works to support teaching at the university by delivering inclusive and innovative workshops and programming for anyone wishing to improve their teaching skillset. The programming can focus on foundations of teaching in higher education, course design, educational technology, and acces-
sible teaching. Certificates gained through the TATP can be listed on your CV.
Participating in local conferences. Many conferences have high associated costs and funding support is not always available, however the SGS Conference grant offered by the School of Graduate Studies is an excellent resource to boost participation. It is often awarded to junior graduate students to support early career networking and it’s highly recommended that students take advantage of this early on in their degree. Attending local conferences held at or around the University of Toronto is a useful strategy to improve upon this aspect of your CV. The Department of Immunology, the Emerging & Pandemic Infections Consortium, and the Beyond Sciences Initiative all hold annual conferences where graduate students and postdoctoral fellows can present their findings either as an oral or poster presentation. Monetary awards are also given to the most highly regarded presentations.
Ultimately, many opportunities exist to improve your CV, all of which are dependent on your interests and future career goals. However, many of these opportunities require you to investigate them yourself as general information is not always easily accessible. Despite this, communicating your interests with senior graduate students, postdoctoral fellows, and PIs in the department can be a beneficial starting point in learning about the various options available and finding the ideal opportunities that can help you build a competitive CV to achieve your future goals.
Written & Designed by
Baweleta Isho
Surviving as a PhD Student in a Shaky Economy

It is a strange time to be in graduate school. Between inflation, trade disputes, and the rise of artificial intelligence (AI), the world feels like it is shifting faster than any of us can keep up. Many young adults are also facing one of the toughest job markets seen in decades. Statistics Canada reports that youth unemployment rate has reached its highest point since the mid-1990s, excluding the pandemic years. People in industry talk about hiring freezes, rescinded offers, and the growing number of temporary or contract positions.
And yet, being a PhD student might be one of the few relatively stable po sitions right now. Our stipends are not extravagant, but they are con sistent. We are not competing for unstable entry-level jobs or participating in the corporate restructuring roulette. Instead, we have something precious: time. Time to learn, to think deeply, and to prepare for whatever this evolving economy becomes next.
Financial Realism Without the Doom
Let’s be honest. The current high cost of living is brutal. Cost of groceries, rent, and transit all seem to rise faster than our income. But financial stability begins with awareness, not despair. Track your spending carefully. Pay off high-interest debt before it snowballs. Build an emergency fund that covers at least three to six months of expenses. These small, consistent habits create independence.

Many graduate students also find creative ways to supplement their income. Teaching assistantships, tutoring, or freelancing in writing, editing, or data analysis can help. The goal is not to chase every dollar but to build flexibility and confidence. A PhD demonstrates that we can think independently, learn quickly, and create new solutions from scratch. Those abilities are not niche skills. They are exactly what the modern economy values most.
AI: The Friend, the Foe, and the
Future Labmate
Nothing dominates the current conversation more than AI. Some people see it as the end of research. They imagine postdoctoral work being automated and the already narrow academic ladder collapsing completely. It is true that the traditional academic model is under strain. The path from PhD to tenure has become much narrower and more uncertain.
However, AI might not be replacing us scientists. It might be freeing us. Imagine a future where algorithms handle data processing and literature searches, while human researchers focus on creativity, interpretation, and discovery. Calculators did not make mathematics obsolete. They changed how we approach it. In the same way, AI will reshape how we conduct science without eliminating the need for human curiosity and judgment.
If you are genuinely passionate about your field, do not let the headlines discourage you. Keep following the questions that excite you most. The world will still need researchers who can ask meaningful questions and interpret complex results in context. Those skills cannot be automated.


Perspective
The Bigger Picture
Economist Armine Yalnizyan recently argued that Canada has all the ingredients to become one of the best economies in the world, if public policy supports investment in healthcare, infrastructure, and science. That is not an abstract idea. It is a call to action.
For researchers, this means more than survival. It means participation. Whether through science communication, policy engagement, or mentoring, we can help shape what comes next.
A PhD is more than a degree. It is a mindset. We are trained to face uncertainty with curiosity and to turn confusion into discovery. In an unpredictable economy, that ability is not a weakness. It is our greatest strength.
Written & Designed by
Tianning Yu
The endless possibilities beyond
Perhapsyou have just started graduate school, or perhaps you are almost done. Regardless of when you started or why you chose this path, at some point you may wonder, “What’s next?”
Maybe by this point, you have decided that you do not want to do wet lab work for the rest of your life. Where do you start? It is not an easy task for graduate students to explore alternate career options. With little information easily accessible and the constant touting of the glory of academia, it is often suggested that either post-doctoral training or research in industry are the primary and most reachable options. However, these options are not suitable for everyone and stepping away from wet lab research does not mean you cannot put your degree to use in your career. There are many ways to leverage your scientific background outside of the lab. According to a 2023 study published in eLife, over 30% of PhD graduates at the European Molecular Biology Laboratory end up in careers outside of wet lab research. We forget there is life outside of the laboratory. We forget we have transferrable skills beyond pipetting and making buffers. There are ways to make a living in science without conducting research, which you can and should explore to figure out which of these options fit your goals and preferred lifestyle. Even early in your graduate studies, taking time to explore your goals and options allows you to make the most of your degree to gain additional transferable skills and strengthen your resume for seamless transition into your desired career.
Although not an exhaustive list, here are some popular non-academic career options you could explore after your graduate degree.
Scientific communication One of the most popular science-related non-research careers, scientific writers are essential to making scientific concepts and results accessible to the general public. This role provides the unique opportunity to contribute to disseminating valuable information and combating misinformation while still staying closely connected to reviewing data. This field extends to journalism, illustration, and even publishing. Since the COVID-19 pandemic, remote and hybrid work arrangements have become common for scientific writers, which may be suitable for your preferred lifestyle.
Consulting Making use of your research expertise, consulting allows you to apply what you have learned to help others looking to succeed in their research. Using your critical thinking skills, you will work in teams to conduct market research, scope client problems, and present strategies for product launches, manufacturing, clinical trials, and other areas along the product pipeline. This job often requires a PhD and some experience in consulting. If you are interested in this field, participating in case competitions would not only provide you consulting experience but begin to build your resume for transitioning into this career.
Regulatory Affairs Like quality control, regulatory affairs require strict adherence to regulatory guidelines for safety and therapeutic efficacy, and an understanding of the laws governing the business. Employers range from biopharmaceutical companies and contract research organizations to government positions. A certification in regulatory affairs can enhance your qualifications and competitiveness for these positions.
Regulatory Affairs
Consulting
Medical Liason
beyond traditional wet lab
Quality Control/Assurance For any product on the market, the manufacturing process needs to be perfected, upscaled, and sustained. This requires a team of experts in monitoring the manufacturing process and assessing the quality of the products through rigorous testing. As a quality control analyst, your role will require you to be meticulous in recording keeping and adhere to strict standards.
Technical specialist/Field application scientist Like consulting, these careers involve applying your knowledge to help others succeed in their research. These roles often involve product demonstrations and troubleshooting from reagents and protocols to hardware installation. If you enjoy traveling and interacting with customers, this career may be the right choice for you.
Medical Science Liason MSLs are employees for biopharmaceuticals that serve as a non-promotional delivery route of drug information to healthcare professionals, such as doctors, nurses, and pharmacists. As an MSL, you would share key trial results, discuss unmet medical needs, and gather insight and feedback on therapeutic regimens and patient care. This career enables you to stay current with clinical research while building strong networks in the healthcare system.
Project Management The demand for project managers continues to grow across different STEM fields. Requiring strong organizational and communication skills, project managers are responsible for planning and resource allocation to ensure product pipelines are executed within scope, on time, and within budget. Acting as the point guard on a basketball team, the project manager coordinates harmonious play and adjusts approach in real time for successful project outcomes. Project management certification courses are offered in many institutions. While not mandatory, the certification program can provide additional skills and insight for effective project management in industry.
Business Development Managers Typically focused on commercializing new ideas or business opportunities, business development managers aim to expand the company’s market presence, drive business growth, and extend partnerships. Working closely with data analysts, business development managers scope competitors, conduct market research, analyze scientific and commercial data, and perform strategic planning to advance company growth. Although not required, a certification in project management or marketing could boost your expertise and competitiveness for this field.
The most challenging part about searching for alternative career paths is that you have limited information and experience in these jobs. Seeking connections and networking with professionals pursuing those careers is critical to not only determining if a job might be the right fit for you but can also help you transition into your chosen career. Just remember that career paths do not have to be linear and there is no wrong choice, we all must start somewhere.
Written by Siu Ling Tai
Designed by Preya Patel
Book Review: Doctored (2025) by Charles Piller
Spending days, weeks or even months working on an experiment, aiming for perfect results makes science feel like art. Just as painters obsess over every brushstroke to create a masterpiece, scientists execute protocols meticulously to generate data that is clean and befitting of their expectations. This is especially true when the results are visual. Magnified and annotated images of brain, lung, liver and stomach tissues reveal what our cells and proteins are up to. These images are more than data; they’re canvases of our biological reality that shape how we understand health, develop treatments and deliver care.
But what happens when these glimpses into the human body are altered to tell a desired story, not our reality?
Charles Piller’s Doctored answers this question. As a self-described look into “Fraud, Arrogance, and Tragedy in the Quest to Cure Alzheimer’s,” Piller investigates how manipulated data—specifically digitally doctored images—has distorted Alzheimer’s research for decades. The cost: misguided drug development, wasted resources and a betrayal of trust among patients, caregivers and clinicians.
Alzheimer’s disease is a devastating neurodegenerative condition that affects over seven million Americans. It damages the brain tissues, impairing cognition, causing memory loss and stealing one’s sense of self. Despite haunting humanity for centuries, with Aristotle even describing how it causes “memory and love to cease,” and billions spent by governments and pharmaceutical companies, progress towards a cure has been painfully slow.
In 2022, Piller published an exposé titled “Blots on a Field,” which investigated Simufilam, a drug developed by Cassava Sciences that was seeking approval for late-stage clinical trials. The company claimed Simufilam blocked the formation of sticky amyloid deposits and reduced inflammation in brain tissues. The evidence was found in images generated by an experimental technique called Western blot which identifies proteins in biological samples by size and structure. However, Dr. Matthew Schrag, a neurologist and researcher familiar with the technique, voiced concerns over duplicated bands, unnatural edges, odd shadows and inconsistent backgrounds—clear signs the images had been doctored.
Alarmed by the possibility the drug might be approved based on fraudulent data, Schrag filed a complaint with the FDA. He then was recruited by Piller to uncover widespread image manipulation across the Alzheimer’s field. The investigation led them to a worrisome figure in Alzheimer’s litera-
“It’s hard to ignore what’s happening when it involves patients who have put a lot of trust in those who run clinical experiments.”
ture: Dr. Sylvain Lesné.
At the heart of the stagnation, Piller argues, is the amyloid cascade hypothesis: the idea that sticky plaques of a protein called amyloid-beta trigger inflammation in the brain, leading to neuronal cell death and cognitive decline. Drugs like Aduhelm and Leqembi were approved by the FDA based on their ability to remove these plaques. However, both have shown minimal cognitive benefit, and have been linked to serious side effects, including brain swelling and bleeding. Despite these disappointing outcomes, the amyloid hypothesis continues to monopolize the field’s funding, drug development and clinical priorities.
In 2006, under the supervision of renowned Alzheimer’s researcher Dr. Karen Ashe at the University of Minnesota, Lesné published a paper in Nature claiming to identify a novel amyloid-beta protein that directly impaired memory in rats. The paper went on to be widely cited across the field and solidified the amyloid hypothesis. But Schrag found signs of result-changing image manipulation throughout Lesné’s works—most worryingly in highly cited articles promoting treatments targeting the new protein. Nobel laureate Dr. Thomas Südhof noted the implications of the revelations: “the immediate, obvious damage is wasted [...] funding and wasted thinking in the field.” Piller stresses that entire careers in research have been built on faulty findings. Meanwhile, promising alternative hypotheses were sidelined and patients were exposed to clinical trials based on fraudulent data, raising serious ethical and safety concerns.
To assess the scale of the prob lem, Piller and Schrag, along side forensic image analysts, launched a follow-up investi gation titled “Other Blots.” They flagged 571 papers with suspected image doctoring, collectively cited over 77,600 times, including 487 citations in active patents. These papers had a significant influence on the direction of Alzheimer’s research and clinical decision-making, proving how misconduct at the bench can ripple outward into industry and patient care.
But exposing widespread fraud comes at a cost. Schrag voices con cerns over junior scientists listed on suspect papers having their careers de railed, even if they weren’t responsible for the misconduct. It’s a consequence of being “in the wrong place at the wrong time,” Schrag explains. If these students were to speak up they may face retaliation by influential figures in the field. Additional ly, although Dr. Ashe was not engaged in data manipulation, she faced years of dwindling fund ing, support and emotional security following the aftermath of her collaborator, Lesné’s, public down fall. She said “It was like losing a limb [...] I lost part of my reputation. It will never grow back.” Piller also recalled how a friend of his warned that too much mis conduct coverage could lead the public to see science as a “cesspool of corruption.”
Another issue Piller says contributes to the problem is that accountability is rare. Universities often conduct year-long investigations only to dismiss accusations. Journals delay retractions. Authors themselves deny wrongdoing without providing evidence. Piller writes, “It’s like a world without police or prosecutors.” But the importance of speaking up doesn’t escape Piller or Schrag, with Schrag concluding “It’s hard to ignore what’s happening when it involves patients who have put a lot of trust in those who run clinical experiments.”
“You can’t cheat to cure a disease. Biology doesn’t care.”
Despite its sobering revelations, Piller offers some hope. Following the scrutinization of the amyloid hypothesis, new directions in Alzheimer’s research are emerging, including research into the role of viral infections and the potential of weight-loss drugs to reduce brain inflammation. To continue moving the field forward, Schrag suggests stronger publishing oversight and a cultural shift away from the “publish or perish” mentality. This may discourage the submission of manipulated findings and the adoption of a single unopposed theory in the field. Ultimately, Doctored reminds us that what happens at the bench has the potential to affect the course of people’s lives. And while science, like art, is shaped by human hands, it is judged by truth, not beauty. As Schrag says, “You can’t cheat to cure a disease. Biology doesn’t care.”
Written by Yasmin Anning
Designed by Angelica Lau
Department of Immunology
University of Toronto
1 King’s College Circle
Toronto, ON M5S 1A8
Canada

Banting and Best’s Laboratory (192-)
Photo
