





JANUARYDECEMBER, 2026
We are first in quality. Our team has been chosen by leading companies. Let us unlock your potential.





Participants will understand what are the 7 QMS Principles and how they can be applied within any organization to promote quality in accordance with ISO 9001:2015; which clauses of ISO 9001 relate to each principle, how to evidence the implementation of each principle within their organization.


Managers,Supervisors,QualityProfessionalsandInternalAuditors.
Thiscoursewillassistparticipantstounderstandtheintentofthe ISO9001standard,whatneedstobedonetobuildanISO 9001:2015system,upgradingthecurrentsystemorintegratingit withanothermanagementsystem,benefitsofimplementingthe qualitymanagementsystemandtheapplicationofthe requirementstoatypicalbusinessenvironment.
February10-12,2026
Thirtythreethousanddollars($33,000)




Management Representatives, Supervisors, Quality Managers and Persons desirous of becoming Auditors.
Internal audits determine the status of your Quality Management System and participants will acquire tools to execute an effective audit process, write intelligible and accurate Nonconformity statements and assess the significance of the findings and prepare the audit report.




Managers,Supervisors,QualityProfessionalsandInternalAuditors.
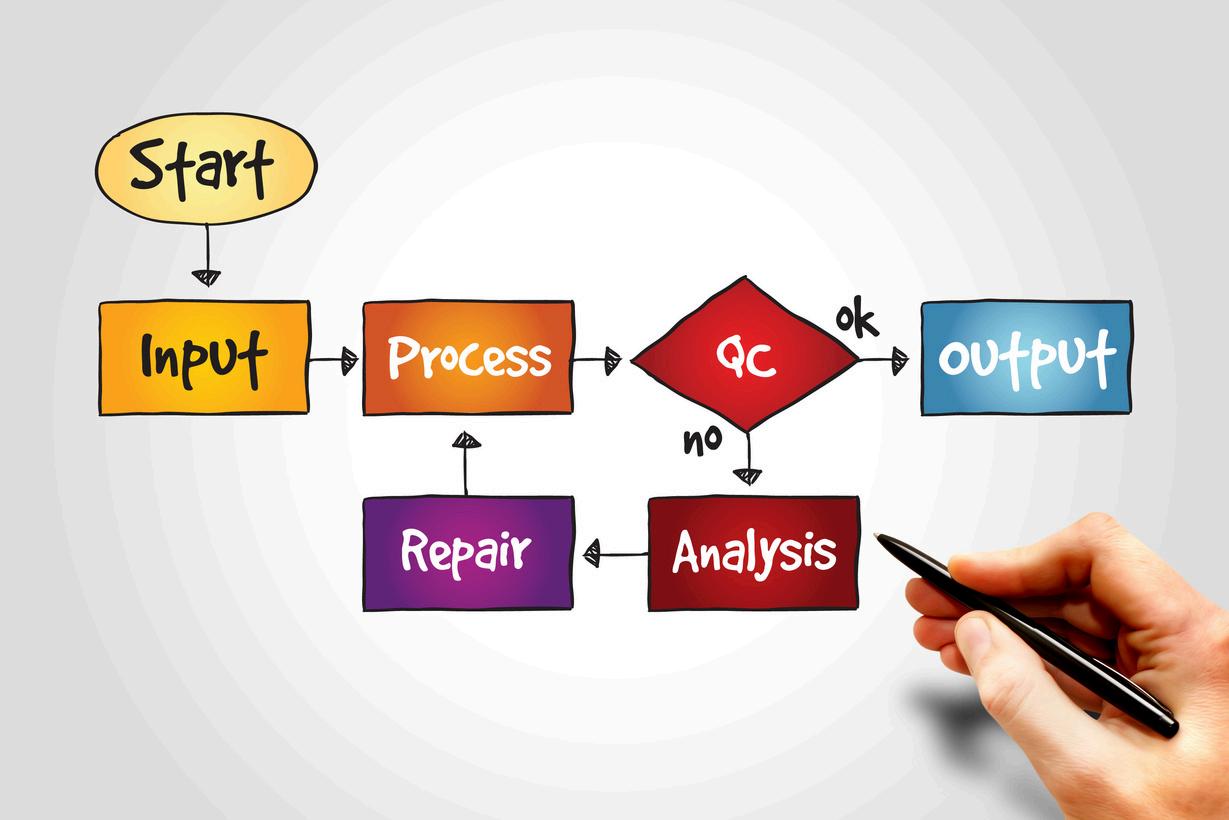

Managers,Supervisors,QualityProfessionalsandInternalAuditors.
Participants will gain practical knowledge of Statistical Process Control (SPC) techniques to monitor, control, and improve process performance. They will be able to interpret and use control charts, analyze process variation, and apply data-driven decision-making to enhance quality, consistency, and continual improvement in line with recognized standards


Qualityassurancepersonnel,productionandinspectionstaff,foodandproduct manufacturers,andanyoneinvolvedinsampling,testing,orensuringproduct conformityandcompliancewithstandards.


Demonstrate how any type of medical, testing, or calibration laboratory can utilize the requirements of the GYS 170:2021 (General requirements for the operations of a laboratory) standard to streamline its operations.


Topmanagementandseniorleadership,qualitymanagersandcoordinators, Processownersandinternalauditors,membersofcross-functionalmanagement teamsresponsibleforsystemperformance
Participants will develop the ability to conduct effective ISO 9001:2015 management reviews, applying a structured, risk-based approach to analyze performance, drive improvement, and support informed decision-making, continual improvement, and enhanced organizational performance and customer satisfaction.


Foodmanufacturersandoperators,qualityassurancepersonnel, andstaffinvolvedinfoodprocessing,packing,anddistribution.


Quality Managers, Laboratory Supervisors, Internal Auditors, Laboratory personnel of various testing laboratories, Technical staff, prospective assessors and consultants, and auditors of all types who are desirous of improving their knowledge of ISO/IEC 17025.
This course will use the ISO/IEC 17025:2017 standard, General requirements for the competence of testing and calibration laboratories to demonstrate the importance of implementing a laboratory management system to assure the confidence in conducting tests and calibrations, sampling, and providing accurate and reliable test and calibration results. Twentyseventhousanddollars($27,000)


InspectorsorOfficersresponsibleforinspectionactivities,Technical Managers,DeputyTechnicalManagers,InspectionSupervisors.
Participants will develop an understanding of ISO/IEC 17020:2012 requirements for competent, impartial, and consistent inspection activities, and gain practical skills to apply the standard using effective tools and techniques to strengthen inspection agency operations.


This course is designed to provide participants with the required knowledge and tools to interpret and apply this Prerequisite Program which forms the foundation for a food safety system. The course is designed to complement the GNBS HACCP programme and can be attended as a stand-alone course before attendance to HACCP training.


Thiscoursewillassistparticipantstounderstandtheintentofthe UnderstandingtheRequirementsoftheGNBS901Standard, upgradingthecurrentsystemorintegratingitwithanother managementsystem,benefitsofimplementingthequality managementsystemandtheapplicationoftherequirementstoa typicalbusinessenvironment.
May11-12,2026
Twentyseven thousanddollars($27,000)


Managers,Supervisors,QualityProfessionalsandInternalAuditors.






QualityProfessionals,ProductionManagers,Supervisors,ServiceProfessionals andotherTechnicalStaffinvolvedinProductionandServiceProvision.
This two (2) day programme provides participants with an understanding of the principles and concepts of the Estimation of Uncertainty as required by ISO 17025:2017. The course consists of tutor-led presentations, together with exercises, workshops and feedback on these to reinforce what was learnt during the presentations.
June08-09,2026


Participants will gain the knowledge and skills to understand the problem-solving cycle and properly define problems and use appropriate tools and techniques to thoroughly investigate them to determine possible causes and the root cause, and determine the most appropriate solution to the defined problem and test the implementation of the solution.


Participants will understand what risk is and risk-based thinking (RBT) in the context of ISO 9001:2015. They will be able to interpret the requirements of the ISO 9001:2015 that deals with RBT as well as determine the context of the organisation. Finally, participants will finish the course understanding the basic steps and methodology of the overall risk management process, identify QMS risks, evaluate and analyse QMS risks in their organisations and determine risk mitigation and control measures for the QMS.




Health & Safety/HSE Managers/Coordinators, Health & Safety/HSE Officers, Health & Safety/HSE Auditors, Health & Safety/HSE Supervisors and other staff involved in developing, documenting and managing the OH&SMS of their organizations.
At the end of the 3 days, participants will be able to develop and document an Occupational Health and Safety Management System (OH&SMS) or revise their existing OH&SMS documentation to meet the requirements of ISO 45001:2018. The course also enables participants to implement an effective OH&SMS based on the Plan Do Check Act cycle and incorporating legal and other requirements, hazard analysis, risks and opportunities, and operational controls in the OH&SMS.


Management Representatives, Supervisors, Quality Managers, Persons desirous of becoming Auditors.
By the end of this course, participants will be able to understand the fundamentals of auditing practices, evaluate the effectiveness of a company’s management system in achieving their declared quality objectives; understand the follow-up actions needed to verify the effectiveness of an organisation’s implemented improvements and audit organisations’ quality management systems.
Twohundredthirtythousanddollars($230,000)










Participants will be able to plan and conduct internal audits to assess laboratory compliance with GYS 170:2021, identify and report nonconformities, recommend corrective actions, and support continual improvement of laboratory operations and quality
QualityManagers,LaboratorySupervisors,InternalAuditorsandAll levelsofLaboratoryPersonnel. Thirtythreethousanddollars($33,000)
August04-06,2026


Participants will understand the requirements of ISO 15189:2012, enabling them to ensure medical laboratory quality and competence, implement effective quality management systems, identify nonconformities, and support continual improvement in laboratory operations.
QualityManagers,LaboratorySupervisors,InternalAuditorsandAll levelsofLaboratoryPersonnel. Thirtythreethousanddollars($33,000)
August10-12,2026




QualityManagers,LaboratorySupervisors,InternalAuditorsandAll levelsofLaboratoryPersonnel. Thirtythreethousanddollars($33,000)
Participants will understand the requirements of ISO 15189:2012, enabling them to ensure medical laboratory quality and competence, implement effective quality management systems, identify nonconformities, and support continual improvement in laboratory operations.


Managers,Supervisors,QualityPersonnel,Auditors.


Managers,Supervisors,QualityPersonnel,Auditors.
Participants



Laboratory Personnel, Quality Managers, Managers and Supervisors of Businesses and Organisations.
Participants will be able to understand the different types of data and their use and applications; proactively plan the data collection exercise or use data collected for other purposes; understand the benefits of data collection and analysis within their organizations for program implementation, providing summary information for management reports and as a basis for continual improvement, etc.


Managers,Supervisors,QualityProfessionalsandInternalAuditors.
Thiscoursewillassistparticipantstounderstandtheintentofthe ISO9001standard,whatneedstobedonetobuildanISO 9001:2015system,upgradingthecurrentsystemorintegratingit withanothermanagementsystem,benefitsofimplementingthe qualitymanagementsystemandtheapplicationofthe requirementstoatypicalbusinessenvironment.
October26-27,2026
Thirtythreethousanddollars($33,000)


QMSManagers/Coordinators,QMSAuditors,Supervisors,Staff involvedindeveloping,documenting,andmanagingtheQMSof theirorganization.
This course enables participants to develop and document a QMS or revise their existing QMS documentation utilizing the process approach and risk-based thinking. The course also enables participants to implement an effective QMS based on the Plan Do Check Act cycle by developing the QMS Policy and Objectives.


Management Representatives, Supervisors, Quality Managers, Persons desirous of becoming Auditors.
By the end of this course, participants will be able to understand the fundamentals of auditing practices, evaluate the effectiveness of a company’s management system in achieving their declared quality objectives; understand the follow-up actions needed to verify the effectiveness of an organisation’s implemented improvements and audit organisations’ quality management systems.
Twohundredthirtythousanddollars($230,000)




Internal audits determine the status of your Quality Management System and participants will acquire tools to execute an effective audit process, write intelligible and accurate Nonconformity statements and assess the significance of the findings and prepare the audit report.








Secure your spot by registering on or before the recommended date.
knowledge of the requirement of ISO 9001: 2015 and ISO 45001: 2018 would be relevant for participants attending the lead auditor and implementing and documenting courses.
Upon successful completion of training courses participants will receive a certificate.