

AUDIT TOOL
UPDATES TO OIG’S AREA OF FOCUS ON CARE AND SAFETY OF NURSING FACILITY RESIDENTS
MEDICAL CODING TOXIC SHOCK SYNDROME
TWO HEALTHCARE ITEMS EMPHASIZING THE IMPORTANCE OF STATE LAWS
NEW MR SAFETY CODES PROVIDE ADDITIONAL REIMBURSEMENT FOR RADIOLOGISTS

AD



NOTE EDITOR’S
Welcome to the “ber” months! Perhaps “brr” is more appropriate—unless you live in the South like me, where it’s still swimming weather! But I grew up in Indiana, where September and October are the greatest months of the year. I hope that wherever you are, you are experiencing cooler days, nights, and breezes. With or without that here in southern Louisiana, I’ll buy all the mums and pumpkins anyway—replacing all that summer brought. Because sometimes, don’t we just need a reason to change things? What things are you willing to let “fall” this season, to allow for growth in the next?
Before I joined BC Advantage, I worked in healthcare for several years, primarily in nursing homes. In one facility, where I worked for three years, I worked with a woman who was the absolute best in her role, inspiring me to be better in my own. I had never met anyone who cared so genuinely and gently for the residents. Quality of care was her driving force—even if that’s not what she called it. To her, it wasn’t even about improving quality of care; it was simply about loving people where they are and treating them like human beings. I was good at my job—a checker of all the boxes—but my friend certainly taught me how to do better and be better. Both professionally and personally. Whether in my own life or for my residents in their home, I learned to let go of some things to focus on quality of care—and thus, quality of life.
and the nursing facility workforce. The OIG has made improving the quality of care and safety of nursing home residents a top priority. Their recent guidance addresses goals to reduce fraud, waste, and abuse; promote cost-effective and quality care; enhance the efficiency of providers’ operations; and propel improvements in compliance and resident safety and care in nursing facilities. And again, it’s not just about meeting guidelines; it’s about treating people the way we all want to be treated. If we can master that, it only improves from there.



coo Nichole Anderson, CPC nichole@billing-coding.com

Our cover this issue is “Nursing Home Compliance Audit Tool: Updates to OIG’s Area of Focus on Care and Safety of Nursing Facility Residents” by Kirsten Taylor-Billups, JD, RN, CHC, owner and operator of Legal Healthcare Consulting. This article emphasizes that quality of care and safety are matters of critical importance for residents, their loved ones,
Other articles in this issue are on the 2026 physician fee schedule, coding pain in the left knee, coding toxic shock syndrome, coding integrity and CAC, guiding vaccination strategies, healthcare items emphasizing state laws, overcoming patient access challenges, advanced life support level 2, new MR safety codes, determining when it’s time to hire a coder, coding Alzheimer’s, and our monthly spotlight on fraud, waste, and abuse (FWA). One FWA case in particular serves as a reminder that, again, both professionally and personally, we should be treating people as human beings.
When you are with your favorite humans, what are your favorite fall activities? Whether you go apple picking/bobbing, play/watch football, or rake/jump in leaves, I encourage you to do it with intentionality. All distractions aside, look at their faces, hear their laughter, and make new, core memories. And take some photos—fall is great for that!
Amber Joffrion BC Advantage Editor
subscriptions manager Ashley Knight ashley@billing-coding.com
advertising sales@billing-coding.com
Renewals: Keep your rate locked in for life! www.billing-coding.com/renewals
Change of address: Email subscriptions@billing-coding.com or call 864 228 7310
ICD-10 Code M25.562 Pain in Left
Knee: When and How to Use
There Might Be Some Good News in the Medicare Physician Fee Schedule Proposed Rule for 2026
Medical Coding Toxic Shock Syndrome
Coding Integrity and CAC: Why Quality Must Precede Compliance In Healthcare Documentation
Guiding Vaccination Strategies for Small Practices Across Specialties
Nursing Home Compliance Audit Tool Updates to OIG’s Area of Focus on Care and Safety of Nursing Facility Residents Two Healthcare Items Emphasizing the Importance of State Laws
Life Support Level 2 (ALS2) 2025 Updates
New MR Safety Codes Provide Additional Reimbursement for Radiologists
Monthly Spotlight on Fraud, Waste, and Abuse Is It Time to Hire a Coder? A Guide For Small Practices
Mastering Medical Coding and Billing for Alzheimer’s Disease: Guidelines and Tips

EXPERT CONTRIBUTORS THIS ISSUE
Dr. Stacey R. Atkins, PhD, MSW, LMSW, CPC, CIGE, is a Compliance Specialist working as a team member in the Education Department of the American Institute of Healthcare Compliance (AIHC). www.aihc-assn.org
Karen Blanchette, MBA, is the Executive Director of PAHCOM. The PAHCOM collaborative network enables solo providers and small group physician practices to access focused information vital to managing their healthcare businesses effectively. https://my.pahcom.com/ certifications.
Ryan Chapin, Executive Director of Strategic Solutions at AGS Health, assists with strategic growth initiatives for the company’s Patient Access and Patient Financial Services business units. www.agshealth.com
Sandy Coffta is Vice President of Client Services at Healthcare Administrative Partners. Coffta has over 17 years of experience in client relationship management, including reimbursement analysis, workflow optimization, and compliance education. www.hapusa.com
Shannon Deconda is the Director of Coding and a DoctorsManagement partner. She is also the founder and president of NAMAS (National Alliance of Medical Accreditation Specialists. https://namas.co/
Janine Mothershed, CPC, CPC-I, is the founder and CEO of Coding Clarified, an innovative online medical coding school committed to transforming lives through flexible, high-quality career training. A Certified Professional Coder (CPC) and licensed AAPC instructor, Janine brings over a decade of experience in healthcare administration, medical coding, and workforce development. Codingclarified.com
Sonal Patel, BA, CPMA, CPC, CMC, ICDCM, is CEO and
THIS ISSUE
Principal Strategist of SP Collaborative, LLC. Sonal has over 13 years of experience understanding the art of business medicine as a nationally recognized thought leader, speaker, author, creator, and consultant to elevate coding compliance education for the business of medicine. www. spcollaborative.net
Providers Care Billing LLC is a leading medical billing services company, specializing in comprehensive revenue cycle management for healthcare providers across the USA. Providers Care Billing is committed to delivering costeffective, reliable billing solutions. www.providerscarebilling.com
Rachel V. Rose, JD, MBA, advises clients on compliance, transactions, government administrative actions, and litigation involving healthcare, cybersecurity, corporate, and securities law, as well as False Claims Act and DoddFrank whistleblower cases. www.rvrose.com
Erin Stephens, CPC, CIRCC, is the Sr. Client Manager of Education at Healthcare Administrative Partners. Healthcare Administrative Partners (HAP) provides revenue cycle management, clinical analytics, and comprehensive practice management solutions for radiology practices, as well as coding services for multispecialty practices. www. hapusa.com
Kirsten Taylor-Billups, JD, RN, CHC, is the owner and operator of Legal Healthcare Consulting. With 35 years of experience in acute and post-acute care, Kirsten has undertaken various roles, including Director of Nursing, Quality Assurance Consultant, Risk Manager, and Corporate Compliance Officer at multi-facility healthcare organizations, such as University Hospitals, HCR ManorCare, Common Spirit Health, and Catholic Healthcare Initiatives.
We are always interested in hearing from any industry experts who would like to get published in our national magazine. Email us at editorial@billing-coding.com to request a copy of our editorial guidelines and benefits.
ICD-10 Code M25.562 Pain in Left Knee When and How to Use

Knee pain is one of the most confirmed musculoskeletal complaints acknowledged in the U.S., specifically in people over 40 years old and those with osteoarthritis or obesity. Although both knees are susceptible to pain, and research does not show that knee pain in one knee is more common than the other, this article focuses on pain in the left knee.
A proper diagnosis and code, ICD-10 M25.562, of discomfort in the left knee is absolutely necessary in treatment, as well as for reimbursement, and may be because of the cause, such as an acute injury or an overuse issue. The wrong codes or no documentation can cause delays, denials, or even an audit, which is detrimental to your pocket and your patients.
What Does Knee Pain Mean?
Knee pain refers to any discomfort or pain felt in or around the knee joint. While experienced more in older adults, pain in the knee is a common concern among individuals of all ages. Knee pain may be due to a traumatic injury involving a torn ligament or damaged cartilage, and in such a case, the knee pain likely needs a separate ICD-10 code. Additionally, knee pain can be a side effect of disorders like gout, arthritis,
and infections. It may also stem from sciatica, osteoarthritis, or obesity.
Self-care measures are effective in treating many varieties of minor knee pain, such as resting and elevating the knee, alternating ice and heat, using compression, taking mild pain relievers, and massaging or performing gentle exercises to relax and strengthen the surrounding muscles. Knee pain can also be treated by physical therapy and knee braces. Sometimes, surgery is required to repair the knee.
Symptoms
The cause of the knee pain is the determinant of the area and intensity of the pain.
The following are general symptoms that may accompany knee discomfort:
Stiffness and swelling
Warmth and redness at the touch
Instability or weakness
Sounds of popping or crunching
Not being able to fully extend the knee
ICD-10 Diagnosis Code of Left Knee Pain
The appropriate code for left knee pain is ICD-10 M25.562 - Discomfort in the left knee. The code falls under the group M25.5, where all pains in joints are included within the specified anatomical areas. It is necessary to utilize the billable ICD-10 code M25.562 to indicate that therapy is sought for left knee pain, since it is the code that most appropriately depicts this sort of discomfort. Due to their ambiguity, more generic codes such as M25.5 can cause a denial of claims, especially when the pain is chronic. To review ICD-10 codes for left knee pain, see Table 1.
Table 1
ICD-10 Codes for Left Knee Pain
Code Description
M25.562 Left knee pain
M23.262 Chronic instability in the left knee
M17.12 Primary osteoarthritis left knee unilateral primary osteoarthritis
M17.32 Left knee osteoarthritis post-traumatic unilateral
M22.42 Patellar tendinitis in the left knee
M23.52 Current meniscus derangement, left knee
Using M25.562 Properly for Billing
In case the results of the patient coincide with the absence of a more definite diagnosis, left knee pain, M25.562, applies. In their documentation, healthcare practitioners ought to give detailed descriptions of the pain and the outcome of any imaging scans or physical examination, together with the pertinent ICD-10 codes. Left knee pain ICD-10 code use is not only a matter of compliance but is also essential for accurate diagnosis and treatment.
Accurate coding has a direct effect on
Insurance reimbursement
Proper care of the patient
Clinical accuracy
Audit preparedness
Case Study
A 35-year-old marathon runner who has been complaining of chronic discomfort in her left knee is seen by a sports medicine specialist in Texas. Clinical notes on pain during jogging, limited motion, and swelling are included in her chart. Mild osteoarthritis and tendon irritation are seen on MRI. The insurance paid for diagnostics and physical therapy, thanks to proper coding using M25.562, M17.12, and CPT codes for imaging and evaluation, ensuring accurate diagnosis.
There may be times when M25.562 is not appropriate. Remember to code to the highest level of specificity to avoid denials. See similar and related ICD-10 codes in Table 2.
Table 2
Similar and Related ICD-10 Codes
Code Description
M25.561 Right knee pain
M25.569 Unspecified knee pain
M22.2X2 Syndrome of patellofemoral pain often associated with bilateral knee pain
M25.369 Other uncertain knee instability
M25.869 Additional specific joint conditions
Common Mistakes in Coding Left Knee Pain
To avoid making these common mistakes, remember the following tips:
Issue of Using Unspecified Codes: Do not use such codes as M25.569 (unspecified knee) when the laterality is clear.
Not Connecting ICD-10: Anticipate that diagnostic (ICD-10) and procedural (CPT) codes are complementary and essential for accurate documentation.
Missing Comorbidities: Record comorbid conditions, such as obesity (E66.9) or trauma, which may change the treatment.
Obsolete Coding: Analyze the recent versions of ICD-10 annually.
The Significance of ICD-10 in Healthcare
The ICD-10 system is a complete language for healthcare professionals worldwide—not just codes. Such uniformity facilitates tracking of health data and patterns, which are vital in research and health policies. Standardization of diagnoses with the help of codes like M25.562 makes the handing over of healthcare-related projects between scholars and professionals easy on an international level.
Insurance and Billing With ICD-10: The Role
Accurate ICD-10 coding is necessary in order to generate insurance reimbursement and billing processes. Such codes as M25.562 will ensure that those working in the medical field will be properly rewarded with fair payment for their



services. Also, accurate coding will help reduce the risk of auditing and denying services, which will make the financial part of healthcare delivery an easier task. Translating patient care into financial viability contributes to the sustainability of healthcare practices.
Conclusion
Correct diagnosis and coding of left knee pain, ICD-10 code M25.562, is important when providing highquality care, as well as ensuring that your practice is paid. When used with correct CPT codes, detailed transcription, and knowledge of the intricacies of related conditions, it can have a vast effect on the success of your billing. Whether with an orthopedic provider, a sports medicine professional, or a general practitioner, accuracy in coding and billing is not an option.
Providers Care Billing LLC is a leading medical billing services company, specializing in comprehensive revenue cycle management for healthcare providers across the USA. Providers Care Billing is committed to delivering cost-effective, reliable billing solutions. Contact them today for a free consultation and learn about their industry-leading healthcare billing services.
providerscarebilling.com

THERE MIGHT BE SOME GOOD NEWS IN THE MEDICARE PHYSICIAN FEE SCHEDULE PROPOSED RULE FOR 2026

The Proposed Rule issued by the Centers for Medicare and Medicaid Services (CMS) for payments under the 2026 Physician Fee Schedule (PFS) contains an increase from the current 2025 rate. There will be two different fee schedules determined by a provider’s participation status in Alternative Payment Models (APM). The Conversion Factor (CF) in the 2026 Proposed Rule is $33.5875 (up 3.83%) for Qualified Professionals (QPs) or $33.4209 (up 3.32%) for non-QPs, compared with the $32.3465 currently in use.
Both CF calculations apply a positive 0.55% budget neutrality factor and a 2.50% increase that was contained in the budget bill (One Big Beautiful Bill Act or OBBBA) passed in July, to arrive at a baseline CF of $33.3375. However, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA 2015) contained a requirement that participants in APMs would receive an additional 0.75% annual update beginning in 2026, while non-APM participants would receive a 0.25% annual update. Thus, the 2026 CF proposed for QPs becomes $33.5875 and for non-QPs becomes $33.4209.
The published CMS estimates indicate that diagnostic radiology and nuclear medicine will be negatively impacted (-2% and -1%, respectively) by the PFS rule, but interventional radiology would see a 2% increase. The rate of impact varies significantly depending on the site of service, as follows in Table 1.
Valuation Changes May Offset the Rate Increase
TThe MPFS contains language that shifts reimbursement away from services provided in a facility (hospital) setting
Table 1
and toward office-based services. The proposed rule would “reduce the portion of the facility PE RVUs allocated based on work RVUs to half the amount allocated to nonfacility [practice expense] PE RVUs”. This would create a redistribution of value from facility-based services (typically hospitals) to non-facility-based services such as imaging centers or Office Based Labs (OBL). However, the Federal Affairs Committee of the Radiology Business Management Association (RBMA) has learned that this Site of Service Differential will not apply to diagnostic radiology services billed with Modifier -26. Thus, hospital-based radiology will not be negatively impacted but it seems that office-based services could benefit.
CMS also proposes to apply an Efficiency Adjustment to
the work RVU and corresponding intraservice portion of physician time for non-time-based services. As proposed, this would generally apply to all codes except time-based codes such as Evaluation and Management (E/M) services.
“Included code families represent the procedures, diagnostic tests, and radiology services that CMS expects to accrue efficiencies over time as changes in medical practice occur, including changes in clinician expertise, workflows, and technology”, according to CMS.
For radiology practices, some of these adjustments could offset much of the increase in the fee schedule CF. We will continue to analyze which procedures would be affected and assess the impact to radiology.
- If you are out of state temporarily (e.g., vacation), then the Medicare rules do not apply. You should still investigate the medical-legal aspect of state licensure in that circumstance.
Expiring Regulations
A Continuing Resolution (CR) passed by Congress in March 2025 extended the 1.0 work floor factor that is used to calculate the geographic adjustment of the Medicare fee schedule through September 30, 2025. This Geographic Adjustment Factor (GAF) is mentioned here as it is scheduled to expire and could impact radiology practices’ reimbursement before the end of 2025 if it is not extended. There is no permanent change to the 1.0 work floor in the Proposed Rule for 2026. Refer to our article What Else is in the Medicare Proposed Rule for 2025? for a full explanation of the GAF.
Here are some examples using a hypothetical practice located in Massachusetts:
• You work and live in Massachusetts; you may read and create final reports from home.
• You work in Massachusetts but live in New Hampshire; you may create final reports from home if you have a New Hampshire license and notify the billing team of the reading address. You may create preliminary reports from home if the final is signed off from the hospital.
• You live and work in California reading exams done in Massachusetts; you must have a license in both California and Massachusetts and notify the billing team of your reading address.
Supervision Requirements
• You work and live in Massachusetts but are on vacation in Cape Cod, MA; you may create final reports. There is no need to notify billing.
The MPFS Proposed Rule would make permanent the supervision flexibilities granted in 2020 during the COVID-19 pandemic that are due to expire at the end of 2025. Physician offices and IDTF’s would be able to directly supervise certain diagnostic tests, such as Level 2 contrast administration using real-time audio and visual interactive telecommunications technology. Note that both audio and video are required; audio-only connectivity is not sufficient. However, as Tom Greeson of ReedSmith points out in his recent article, “The excitement in the radiology community for this proposal is tempered by recent statements made by the Drugs and Contrast Media Committee of the American College of Radiology (ACR) as well as the American Society of Radiologic Technologists (ASRT) at its the Annual Governance and House of Delegates Meeting.” Those organizations are recommending that supervision should include an on-site licensed practitioner.
• You work and live in Massachusetts but are on vacation in Florida; you may create final reports but check on the legal aspect of licensure. There is no need to notify billing.
Some practice systems might automatically capture reading location, but in the end, it is the physician’s responsibility to notify the practice about their work location. Making them aware of these guidelines, especially as they relate to medical licensure, will
Interventional radiology would have 6 MIPS quality measures and 4 QCDR quality measures, plus 19 improvement activities and 3 cost measures. The Society of Interventional Radiology (SIR) is concerned that “the [MIPS Value Pathway] does not adequately reflect the diversity and highly subspecialized nature of IR.”
The easy availability of remote reading and the post-pandemic shift to off-site work has renewed the need for practices to be aware of the Medicare rules for reporting the place of service in order to be compliant. After-hours reading by radiologists in distant locations will produce a reimbursement rate different than the one in effect at the imaging center or hospital. In some cases, this can be used strategically to move interpretations to a location with higher reimbursement rates. The first step is to develop a system that allows the billing team to know the location of the reading services, so they can be sure to apply the rules properly.
Table 1: See below
“SIR believes the measure set is too narrow, making it difficult for many IRs to participate meaningfully without undue burden.” SIR indicates that only 3 measures are broadly applicable across the specialty, with “the remainder primarily apply to interventional radiologists subspecializing in stroke care, dialysis access, venous services and women’s health.”
Most of the other aspects of the MIPS rules would remain unchanged for 2026, including the MIPS Performance Threshold which is proposed to remain at 75 points through performance year 2028.
Conclusion
Sandy Coffta is Vice President of Client Services with Healthcare Administrative Partners. In this role, Sandy oversees the team responsible for achieving and maintaining the company’s consistently high retention and referral rates. Ms. Coffta has over 17 years of experience in client relationship management, including reimbursement analysis, workflow optimization, and compliance education. She specializes in business intelligence and reporting development, is a subject matter expert in radiology practice billing, and has deep expertise in resolving payor disputes and contract issues. www.hapusa.com
Payment Locality Reporting for Radiology Professional Services
The Final Rule often mirrors the provisions laid out in the Proposed Rule with little change. The CF is typically modified slightly due to final calculations being applied, but there should be no significant difference. The controversial Efficiency Adjustment of 2.5% to “non-time-based procedures” is already causing a lot of discussion among physician advocacy groups, which could ultimately influence CMS’s final decision.
Hospital IP, OP, ER
Hospital IP, OP, ER Different
Imaging Center Different Imaging Center
Imaging Center Different
Imaging Center Same Imaging Center
Quality Payment Program
In addition to fee schedule changes, the Medicare PFS covers rules that govern the Quality Payment Program (QPP). The biggest news for radiology is that 6 new MIPS Value Pathways (MVP) would be added, including pathways for both diagnostic and interventional radiology. The Proposed Rule includes 6 MIPS quality measures and 3 QCDR quality measures, plus 11 improvement activities and one cost measure in the diagnostic radiology MVP.
Apart from the MPFS, physicians will be impacted by the changes to coverage under Medicaid and Affordable Care Act (ACA) policies that were included in the OBBBA budget bill. When individuals lose their coverage for whatever reason, practices end up providing more uncompensated care through emergency room visits.
Radiologist’s Office
Imaging Center
Radiologist’s Office
Imaging Center
Imaging Center Same Office in Same Payment Locality Global Imaging Center
CMS is accepting comments on the proposed rule until September 12, 2025. We will provide our analysis of the Final Rule when it is issued.
Imaging Center Same Office in Different Payment Locality Split PC/TC Radiologist’s Office
Note: “Office” includes any location where the radiologist regularly works, which could include his or her home. “Imaging Center” includes a physician office or ASC setting. A vacation hotel or other temporary location should not be reported; the address of the radiologist’s regular work location should be reported in Box 32.
Sandy Coffta is Vice President of Client Services at Healthcare Administrative Partners. Coffta has over 17 years of experience in client relationship management, including reimbursement analysis, workflow optimization, and compliance education. www.hapusa.com 12 BC Advantage Magazine www.billing-coding.com



medical c oding Toxic Shock Syndrome

Toxic Shock Syndrome (TSS) iS a rare buT poTenTially life-ThreaTening condiTion of Ten cauSed by bacTerial ToxinS. iT requireS prompT diagnoSiS, TreaTmenT, and accuraTe medical coding To enSure proper billing and reimburSemenT. ThiS arTicle ouTlineS key coding guidelineS and helpful TipS for documenTing and coding TSS correcTly.
What is Toxic Shock Syndrome?
Toxic shock syndrome is most commonly associated with Staphylococcus aureus or Streptococcus pyogenes infections. It can result from tampon use, skin wounds, or surgical incisions. Symptoms include high fever, rash, low blood pressure, and multi-organ involvement.
TSS symptoms typically appear suddenly and worsen rapidly, often within a few days. In some cases, symptoms can develop as quickly as 12 hours after a surgical procedure or within 3 to 5 days for menstruating individuals using tampons or menstrual cups. TSS can lead to organ failure and death within days.
Swimming in dirty water can absorb through the string of a tampon, and the bacteria in the water can cause a staph infection, which can lead to toxic shock syndrome.
Here’s a more detailed breakdown
Rapid Onset: TSS symptoms, such as high fever, low blood pressure, vomiting, diarrhea, and a sunburn-like rash, tend to appear suddenly and escalate quickly.
Menstrual-Related TSS: For menstruating individuals using tampons or menstrual cups, symptoms can develop within 3 to 5 days.
Surgical-Related TSS: TSS following surgery can manifest as quickly as 12 hours post-procedure.
Importance of Prompt Treatment: Due to the rapid progression of TSS, prompt diagnosis and treatment
Potential for Rapid Deterioration: Without treatment, TSS can lead to severe complications, including organ failure and death, within a short period, sometimes within 48 hours.
ICD-10-CM Coding for Toxic Shock Syndrome
Primary Code: Use A48.3 – Toxic Shock Syndrome when documentation clearly supports a diagnosis of TSS caused by either staphylococcal or streptococcal bacteria.
Additional Codes (if applicable): Use B95.6 (Staphylococcus aureus) or B95.5 (Streptococcus pyogenes) as secondary codes to identify the infectious agent, if documented.
Symptom Codes: Include relevant symptom codes (e.g., R50.9 for fever, R21 for rash) only when they are not integral to the condition or necessary for medical necessity.
CPT Coding and Billing Tips
Inpatient Management: TSS often requires intensive inpatient care. Document all services, such as ICU admission, wound care, or procedures like mechanical ventilation (CPT codes 94002–94004).
Outpatient Follow-Up: Use E/M codes (e.g., 99214, 99215) for office visits addressing recovery or complications.
Lab and Diagnostic Tests: If cultures or other tests are ordered to confirm infection, code separately using appropriate CPT codes (e.g., 87040 for culture, bacterial, aerobic).
Documentation Tips
Ensure provider documentation explicitly states “Toxic Shock Syndrome” and the causative organism, if known.
Note any complications (renal failure, hypotension, respiratory distress).
Be thorough in describing severity, treatment, and site of infection to justify higher-level E/M services or procedures.
Common Coding Mistakes to Avoid
Do not confuse TSS with septicemia or general sepsis unless clearly documented.
Avoid undercoding by omitting secondary diagnoses (e.g., bacterial agent or complications).
Do not code presumptively; always base code selection on confirmed diagnoses or strong clinical evidence.
Key Takeaways
Use A48.3 for TSS and always code for the bacterial agent when available.
Document clearly and completely to support coding, medical necessity, and appropriate reimbursement.
Stay updated with payor-specific rules, as some may require additional documentation for inpatient claims involving TSS.
For more coding and billing insights, visit:
Coding Clarified educational resources and training programs: CodingClarified.com
Toxic shock syndrome (TSS): https://my.clevelandclinic. org/health/diseases/15437-toxic-shock-syndrome are crucial for a positive outcome.
Janine Mothershed is the founder and CEO of Coding Clarified, an innovative online medical coding school committed to transforming lives through flexible, high-quality career training. codingclarified.com
coding inTegriTy and cac: Why qualiTy muST precede compliance in healThcare documenTaTion

Computer-assisted coding, better known as “CAC,” has become the norm over the past decade, but are we producing compliant, accurate results? Compliance begins with quality. In the realm of clinical coding, that means ensuring that documentation tells the full story—and that the codes assigned accurately reflect that story. As CAC becomes more widespread, the need for trained human oversight becomes more critical, not less, which is the reason for this article.
In today’s fast-paced healthcare environment, coding accuracy is often caught in the crossfire between compliance pressures, productivity demands, and evolving technology. While documentation may be clinically sound, coding associated with documentation can be misaligned or inaccurate, particularly when it is generated by CAC tools. CAC can trigger regulatory scrutiny, revenue cycle inefficiencies, and reputational risk without verification by an experienced coder first. As a compliance specialist and educator, I contend that quality cannot be compromised for speed or convenience. In fact, quality is the cornerstone of compliance.
Healthcare consultants recently noted that “documentation is often accurate, but the coding is not,” underscoring a critical gap in the way organizations approach their revenue cycle and risk management. This article explores the current landscape of coding discrepancies, the limitations and risks of CAC, and the essential need for robust internal review processes.
The Disconnect Between Documentation & Coding
In many provider organizations, clinical documentation accurately reflects the patient’s story—diagnoses, treatments, and provider decision-making—but coding processes fall short. Coders may misinterpret documentation, overlook nuances, or rely too heavily on automation, leading to miscoded encounters that can have ripple effects across billing, audit, and quality reporting systems. When errors go undetected, the result can be upcoded services, denied claims, compliance violations, and patient safety concerns. According to the Office of Inspector General (OIG), improper payments stemming from inaccurate coding continue to plague the Medicare program, costing billions annually (OIG, 2023).
CAC: A Double-Edged Sword
Computer-assisted coding (CAC) software, designed to improve speed and efficiency, is now a common fixture in health information management. While these systems can process large volumes of data quickly, their reliance on algorithms rather than clinical reasoning poses significant challenges.
Research has shown that CAC tools may struggle to interpret context, such as distinguishing between active and historical conditions, or differentiating provider impressions from definitive diagnoses (AHIMA, 2022). Without skilled human oversight, these limitations result in critical coding inaccuracies. Unfortunately, some healthcare systems mistakenly treat CAC outputs as final codes without sufficient validation.
Quality needs to be the focus to meet compliance standards. CAC should be a tool to enhance human accuracy—not replace it.
Compliance Risks From Coding Discrepancies
Coding discrepancies—particularly those uncorrected in CAC workflows—are not simply operational issues; they are compliance risks. Auditors from CMS, OIG, and commercial payors increasingly target mismatches between documentation and billing codes. These discrepancies may be flagged as potential fraud, waste, or abuse.
Examples of common coding problems that trigger scrutiny include:
Upcoding or downcoding visits that do not align with documentation
Inaccurate diagnosis coding affecting risk adjustment
Use of unspecified or non-supported codes
Failure to reflect clinical severity accurately
The DOJ’s increased enforcement under the False Claims Act often centers on patterns of poor coding oversight. Healthcare entities must demonstrate that they are taking proactive steps to ensure coding integrity.
Ensuring the integrity of clinical coding isn’t just about reimbursement; it’s about compliance, patient care quality, and data accuracy. As healthcare moves toward value-based models, accurate coding supports correct risk adjustment, patient attribution, and performance measurement.
Implementing regular coding reviews, especially of CAC-assisted encounters, is a best practice that healthcare experts recommend. These reviews should be multidisciplinary, involving coding professionals, clinicians, and compliance officers.
They help:
Identify
patterns of misinterpretation or misclassification
Provide targeted coder education and clinical documentation improvement (CDI)
Verify whether CAC algorithms need adjustment or replacement
Quality assurance activities are not optional; they are essential to both ethical billing and regulatory compliance.
Balancing Productivity Pressures With Accuracy
It is well understood that providers are under immense pressure to manage high volumes of patients while fulfilling extensive documentation requirements. These constraints often lead to documentation fatigue and overreliance on templated language or CAC tools. However, automation cannot replace clinical judgment or attention to detail. Coders must be trained to spot subtle inconsistencies and to understand that their role is pivotal in compliance integrity. Likewise, providers need CDI support that makes documentation more efficient and accurate—not more burdensome.
Healthcare leaders should prioritize investments in coder training, CDI collaboration, and coding audits rather than shortcutting review processes for the sake of productivity.

Recommendations for Compliance-Driven Coding Integrity
To address the CAC errors, organizations Routine Internal reviews of focus on high-risk Coder and documentation updates. Review of codes against final.
Real-Time between CDI discrepancies
Compliance-Focused denial trends, improvement.
Conclusion
Compliance begins that means ensuring and that the codes becomes more becomes more Automation cannot Compliance leaders integrity as non-negotiable allow convenience lead the way.
Dr. Stacey Compliance the Education of Healthcare leadership General, Developmental aihc-assn.org





guiding VaccinaTion STraTegieS for Small pracTiceS acroSS SpecialTieS
Vaccinations remain a vital tool for preventing infectious diseases to safeguard patients and communities. For small physician practices across all medical specialties in the United States—especially general practice, our largest audience segment, alongside pediatrics, obstetrics and gynecology (OB/ GYN), allergy/immunology, and others— adapting to recent federal policy changes is critical to delivering high-quality care. As medical office managers, practice administrators, and physicians, you face the challenge of balancing evolving guidelines, patient trust, and operational demands. This article provides practical strategies to support your patient communities, addressing the latest HHS developments while prioritizing evidence-based care.
Recent HHS Vaccination Policy Changes
Since Robert F. Kennedy Jr. became HHS Secretary in February 2025, significant policy shifts have impacted practices across specialties. These changes, aimed at enhancing transparency and public trust, require careful navigation to ensure compliance and patient safety.
COVID-19 Vaccine Recommendations
In May 2025, Secretary Kennedy announced that COVID-19 vaccines are no longer routinely recommended for healthy children aged 6 months to 17 years or healthy pregnant women. The CDC now uses a “shared clinical decisionmaking” model, allowing providers and patients to assess individual risks and benefits. This shift, bypassing the Advisory Committee on Immunization Practices (ACIP), may affect insurance coverage, as private insurers and Medicaid often align with CDC recommendations. The Vaccines for Children (VFC) program’s inclusion of COVID-19 vaccines is also uncertain, potentially increasing patient costs.
General practice and pediatric offices, which handle childhood vaccinations, should carefully record all vaccine discussions, especially when parents choose not to vaccinate, to maintain clear records and support
ongoing patient care. OB/GYN practices must address maternal vaccination questions, as the American College of Obstetricians and Gynecologists (ACOG) supports COVID-19 vaccines for high-risk pregnancies, citing a 61% reduction in infant hospitalizations from maternal vaccination. Allergy/ immunology specialists, often serving immunocompromised patients, may see increased demand for tailored vaccine guidance under this model.
Overhaul of ACIP and Vaccine Outreach
On June 9, 2025, Secretary Kennedy dismissed all 17 ACIP members, citing conflicts of interest, and replaced them for the committee’s June 25–27, 2025, meeting, which addressed vaccines for COVID-19, RSV, influenza, HPV, and meningococcal disease. This move, reported by ABC News and The New York Times, has raised concerns about the expertise of new appointees, with critics like Senator Susan Collins noting limited vetting time.
In April 2025, HHS underwent a major restructuring, with 10,000 staff cuts across CDC, FDA, and NIH, potentially impacting vaccine outreach efforts. Unconfirmed reports on X suggested the dissolution of a team focused on undervaccinated communities, underscoring the need for practices to stay informed through CDC and professional networks like PAHCOM. For example, CDC grant cuts led to the cancellation of 50 vaccine clinics in Texas, affecting access in underserved areas.
These developments signal potential shifts in vaccine recommendations, particularly for specialties like general practice and family medicine, which serve diverse populations.
Other Vaccination Guidance
The CDC’s core recommendations for childhood and maternal vaccines, including the measles, mumps, and rubella (MMR) vaccine, remain unchanged.
Attending pediatric vaccines, essential for general practice and pediatrics, the schedule includes two MMR doses to protect against measles, a highly contagious disease with 1,168 U.S. cases reported in 2025, 96% among unvaccinated individuals (https://www.yalemedicine.org/news/shouldyou-get-a-measles-vaccine-booster).
Regarding maternal vaccines, OB/GYN practices should offer Tdap (27–36 weeks gestation), the maternal RSV vaccine (32–36 weeks), and the influenza vaccine (before flu season) to protect mothers and newborns.
For specialty-specific vaccines, allergy/immunology and internal medicine may prioritize influenza or pneumococcal vaccines for high-risk adults, per CDC guidelines.
A new HHS requirement for placebo-controlled trials for all new vaccines, announced in April 2025, may delay approvals, impacting specialties reliant on emerging vaccines.
Strategies for Small Practices Across Specialties
Small practices face challenges like limited resources, vaccine hesitancy, and policy uncertainties.
Below are strategies to strengthen your vaccination efforts:
1 Enhance patient education.
Vaccine hesitancy, fueled by misinformation and policy changes, is a widespread issue. A 2023 AAP survey found that 54.3% of pediatricians faced misinformation weekly, a trend likely intensified by COVID-19 vaccine shifts.
Initiate early discussions. General practice and pediatric providers should discuss vaccines at well-child visits, OB/GYN providers during prenatal care, and allergy/ immunology specialists for immunocompromised patients. Use AAP or ACOG patient guides for clarity.
Address concerns empathetically. Acknowledge fears about COVID-19 vaccines, then share evidence, like Nirsevimab’s 90% RSV protection or Tdap’s prevention of 5–15 annual infant pertussis deaths.
Leverage trust. Personalized recommendations, across all specialties, boost vaccine uptake.
2 Streamline vaccine delivery.
Efficient workflows are essential.
Secure supplies. Prebook influenza vaccines and Nirsevimab, especially for general practice and pediatric offices. AAP inventory guides can help.
Integrate vaccinations. Administer vaccines during routine visits, such as Tdap in OB/GYN checkups or pneumococcal vaccines in internal medicine for older adults.
Monitor coverage. Verify insurance policies for COVID-19 vaccines under shared decision-making, documenting medical necessity for appeals, particularly for allergy/ immunology patients.
What’s changing:
3 Foster community engagement.
Community ties are a strength.
Host clinics. Organize flu or RSV vaccine events, especially in areas impacted by reduced outreach. General practice and family medicine can lead, while OB/GYN can target pregnant patients, as Dr. Naima Joseph did with RSV clinics. Partner locally. Collaborate with schools or health departments to promote routine vaccines.
• Review your practice’s supervision protocols to align with the new rule.
vaccine documentation and forums to share strategies for addressing hesitancy or policy shifts. Connecting with the PAHCOM community or exploring its Certified Medical Manager (CMM) credential can enhance your practice’s resilience. Visit my.pahcom.com to learn more.
Starting January 1, 2025, physical therapist assistants (PTAs) and occupational therapy assistants (OTAs) in private practice settings will be allowed to work under general supervision instead of direct supervision. This aligns the supervision requirements for private practice PTs and OTs with those working in institutional providers.
What it means for you:
Share outcomes. Highlight successes, like reduced RSV hospitalizations from Nirsevimab, to build confidence.
• Greater Flexibility: This policy gives PTs and OTs in private practice more leeway to structure care delivery, particularly in areas where staffing challenges exist.
4 Stay informed and compliant.
Navigating changes requires diligence.
• Improved Access to Care: Beneficiaries in rural and underserved regions stand to benefit as therapy services can be delivered with less restrictive supervision.
Track CDC updates. The CDC offers training on vaccine storage and communication, with continuing education credits.
• Regulatory Alignment: The change aligns private practice supervision policies with those of institu-
Rely on professional guidance: AAP, ACOG, and the American Academy of Allergy, Asthma & Immunology provide evidence-based resources, especially for COVID-19 vaccines.
Document discussions. Record vaccine conversations, particularly declinations, to ensure compliance.
Accessing Support for Your Practice
Managing vaccination programs in a dynamic environment demands clinical and administrative expertise. Professional networks like the Professional Association of Health Care Office Management (PAHCOM) offer resources for small practices across specialties, including tools to streamline
• Educate PTAs and OTAs on the changes to ensure they understand their responsibilities under general supervision.
Certification of Therapy Plans of Treatment
Medicare has finalized amendments to the certification requirements for therapy plans of treatment, significantly reducing administrative burdens.
Moving Forward
What’s changing:
The vaccination landscape is evolving, with a phase 2 study for a 31-valent pneumococcal conjugate vaccine planned for early 2026 and potential ACIP changes ahead. Your leadership ensures patients receive evidence-based care. By prioritizing education, efficient workflows, and community trust, and leveraging professional resources, your practice—whether general practice, pediatrics, OB/ GYN, or another specialty—can navigate federal shifts while safeguarding public health.
• Signature Requirement Exception: If a written order or referral from the patient’s physician or non-physician practitioner (NPP) is on file, the therapist-established treatment plan does not need a separate physician/NPP signature for initial certification. The therapist must document evidence that the treatment plan was transmitted to the physician/NPP within 30 days of the initial evaluation.
• No Timeline Restriction on Modifications: CMS did not adopt a specific timeframe within which a physician/NPP could modify the treatment plan. Payment will still be made for services rendered prior to any modification, provided all payment
Karen Blanchette, MBA, is the Executive Director of PAHCOM. The PAHCOM collaborative network enables solo providers and small group physician practices to access focused information vital to managing their healthcare businesses effectively. Contact Karen at https:// my.pahcom.com/contact-karen
Trained and professionally certified managers make a difference. Learn more about the CMM and HITCM-PP at https://my.pahcom.com/ certifications
my.pahcom.com/certifications
Earning the HITCM-PP demonstrates to physicians, staff, and patients that you’re equipped to handle the technological challenges of modern healthcare. It’s especially valuable for small practices, where managers often wear multiple hats and can’t rely on a dedicated IT team. Plus, the certification process itself—complete with study guides and practice exams—builds practical knowledge you can apply immediately. For physician owners, having a HITCMPP-certified manager on staff offers peace of mind and a competitive edge in an industry where trust and compliance are paramount.
Conclusion:

(e.g.,
the practice is in
Cybersecurity isn’t just an IT issue; it’s a practice management issue. For medical office managers and physician owners, ignoring it isn’t an option; the risks are too high. By leveraging professional certifications like the HITCM-PP (https://my.pahcom.com/hit), you gain the knowledge and credibility to protect your practice from digital threats. Pair that with PAHCOM’s free training, and you’ve got a powerful toolkit to break down this massive topic into manageable, practice-specific steps.
compliance.
In an era where cyber threats evolve daily, staying ahead means staying informed. Invest in your skills, tap into free















The Nursing Facility Industry Segment-Specific Compliance Program Guidance (ICPG) was published by the Office of Inspector General (OIG) in November 2024 as the first industry-specific guidance since the November 2023 updated General Compliance Program Guidance (GCPG) was published. Improving the quality of care and safety of residents within nursing facilities is a top priority for the OIG. Quality of care and safety are matters of critical importance for residents, their loved ones, and the nursing facility workforce. The Nursing Facility ICPG, together with the GCPG, addresses the government and private industries’ shared goals of reducing fraud, waste, and abuse; promoting cost-effective and quality care; enhancing the effectiveness of providers’ operations; and propelling improvements in compliance, quality of care, and resident safety within nursing facilities.
The Office of Inspector General (OIG) continues to have
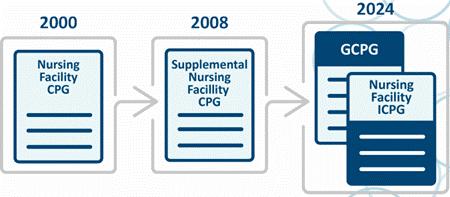
nursing facilities on their radar for compliance guidance and workplans. We’re too far into the year 2025 to say “Happy New Year,” but your nursing facilities aren’t too far along into the new year to revise your compliance program auditing and monitoring tools. In late 2024, the OIG released the Nursing Facility Industry Segment-Specific Compliance Program Guidance (ICPG), which is the OIG guidance for post-acute nursing facilities to update and individualize your compliance programs to improve quality of life, quality of care, risk areas. Together, with the General Compliance Guidance Program (GCPG), nursing can mitigate risks, as well as other important information that the OIG believes nursing facilities should consider when implementing, evaluating, and updating their compliance and quality programs. The OIG demonstrates the migration of compliance guidance for nursing facilities; see Figure 1
In addition to the ICPG’s release, the OIG’s monthly workplan updates should also be considered when updating your compliance programs for your nursing facilities. Upon review of the nursing home focused workplans, you’ll see how they correlate with the topics detailed in the ICPG.
Although the ICPG for nursing homes is not mandatory,
the Requirements of Participation (ROP) for nursing home compliance, modeled after the OIG’s Compliance Guidelines, are mandatory.
Nursing homes must adhere to the ROPs established by the Centers for Medicare and Medicaid Services (CMS) to receive reimbursement for Medicare and Medicaid patients.

The ROPs for nursing facilities include the seven elements that the OIG have identified to promote an effective compliance program:
1 Compliance Policies and Procedures/Compliance Officer are to ensure that all federal and state regulations are being followed;
2 Training/Education of staff on compliance requirements which the policies and procedures are patterned after;
3 Auditing/Monitoring which requires routine audits of billing practices, overpayment rates, and ethics practices, to ensure staff are following compliance and ethics policies and procedures;
4 Reassessments/Modifications to one’s compliance program to address identified weaknesses or changes in regulations;
5 Addressing Quality of Care Issues to ensure care planning is up to date and accurate;
6 Preventing Abuse with the development of policies and procedures to prevent abuse and neglect; and
7 Enforcement of disciplinary mechanisms consistently for violations of compliance and ethics policies/ procedures and regulations.
The implementation of these seven elements can be utilized to mitigate fines, penalties, and enforcements of Corporate Integrity Agreements (CIAs) with the government if violations are identified.
The OIG’s Nursing Home ICPG and workplan recommendations are based upon decades of findings and observations on matters involving nursing homes from audits, investigations, enforcement actions, and monitoring of CIAs. The recommendations are also based upon legal actions and investigations by the OIG current enforcement priorities and interactions with owners, operators, and leaders of nursing homes, trade associations, resident advocacy groups, and other industry stakeholders.
Agencies Working Together
The OIG, CMS, and the Department of Justice (DOJ), as well as other law enforcement agencies, have been working in a concerted effort to pursue nursing facilities that provide grossly substandard care that is being submitted for billing. The government looks at the bills as being fraudulent because the care and services were so inadequate, as if the care and services weren’t provided.
Use the Guidance as a Road Map
The ICPG and workplans both serve as road maps to help nursing facilities focus their efforts on issue selfidentification and corrective actions to minimize the likelihood of civil, criminal, or administrative noncompliance. The ICPG has been written to identify specific categories of compliance risk areas, such as quality of care and life, Medicare and Medicaid billing requirements, federal AntiKickback Statute, and other areas of risk, such as physician self-referrals, anti-supplementation, and privacy and security issues within post-acute care facilities. The list of risks isn’t all inclusive, but it is a good starting point. Facility risk assessments can also be used to formulate audit tools and develop action plans so corrective actions can be implemented and operationalized to demonstrate an effective compliance program—because compliance manuals sitting on a shelf aren’t going to be adequate.
The OIG currently has 13 active workplans targeted for nursing homes, some of which have been active since 2023 and are being revised and brought forward into 2024-2026 compliance OIG reviews. Upon further review of these workplans, they primarily fall into two categories.
1 Skilled Nursing Facilities’ Medicare payments to related parties;
2 SNFs’ billing accuracy of Patient Driven Payment Model (PDPM) reimbursement system;
3 Billing of Medicare Part B services during a Medicare Part A stay;
4 Supplemental payments for Medicaid;
5 SNFs’ financial responsibility for Medicare Part D enrollee(s) medication during Part A stays;
6 Potentially preventable hospitalizations;
7 Employee background checks;
8 Infection prevention and control; 9 Accuracy of reported falls;
Staffing hours; 11 Emergency power systems; 12 Accurate and appropriate reporting; and 13 Usage of antipsychotic medications.
These 13 active workplans should be incorporated into your compliance reviews to some degree, as well as periodic audits to ensure your facility is compliant.
The OIG’s Financial Focus
The OIG’s review of SNF Medicare payments to related parties includes the cost of services, facilities, and supplies furnished to a provider by an organization, related to the provider by common ownership or control. The allowable cost to the provider should only be an amount equal to the related organization’s cost not to exceed the price of comparable services, facilities, and supplies that could be purchased elsewhere.
Key points of the OIG’s review:
Medicare requires that a reported amount be the lower of either the actual cost to the related organization or the market price for comparable services, facilities, or supplies, thereby removing any incentive to realize profits through these transactions.
The OIG’s audit examined whether selected Skilled Nursing Facilities (SNFs) reported related parties as required per 42 CFR 413.17 (d) and whether their related-party costs complied with Medicare requirements. The review is partially complete, and the OIG identified that SNFs weren’t following proper reporting requirements for payments to related parties. Therefore, the OIG made three recommendations to CMS to correct the issues, and CMS adopted two of the three recommendations.
CMS agreed to develop and implement guidance for SNFs on the appropriate methods for providers to determine their allowable related-party costs; and provide guidance to reeducate MACs (Medicare
Administrative Contractors) on the need to review, grant, and document requests from SNFs for exceptions to cost reporting requirements in compliance with 42 CFR §413.17(d).
Given that the OIG has forwarded this area of review into FY 2025, it would be prudent of your own facilities to become familiar with the related parties’ cost report requirements and review your cost reports for allowable related provider cost to determine if your facility is following the regulations. If not, implement an action plan to correct the deficiencies identified.
The other areas of financial focus by the OIG include their review of nursing facility Skilled Nursing reimbursement, Medicare Part B services during a Medicare Part A stay, Supplemental Payments for Medicaid, and SNFs’ financial responsibility for Medicare Part D enrollee(s) medications during Part A stays. These reviews should already be on your annual compliance audit tools to some degree to measure your facility’s level of general billing compliance. However, it does help to narrow your focus and identify the areas being reviewed by the OIG in the above listed areas.
When conducting a review of a skilled facility’s reimbursement, the review can go back as far as 2019 when CMS implemented a new payment system, the PatientDriven Payment Model (PDPM), for determining Medicare Part A payments to skilled nursing facilities. Specifically, CMS implemented the PDPM, a new case-mix classification system for classifying SNF patients in a Medicare Part A covered stay into payment groups under the SNF Prospective Payment System.
Under PDPM, payment is determined by factoring in a combination of six payment components. Five of the components are case-mix adjusted and include a physical therapy component, an occupational therapy component, a speech-language pathology component, a nontherapy ancillary services component, and a nursing component. Additionally, there is a non-case-mix adjusted component to cover utilization of SNF resources that do not vary according to patient characteristics. The OIG will determine whether Medicare payments to SNFs under PDPM complied with Medicare requirements.
When it comes to Medicare Part B services during a Medicare Part A stay, the OIG will determine whether Part B payments to Medicare beneficiaries in nursing homes are appropriate, and whether nursing facilities have effective compliance programs and adequate controls over the care provided to their residents. Medicare pays physicians, nonphysician practitioners, and other providers for services rendered to Medicare beneficiaries, including those residing in nursing homes.
Keep in mind
Most of these Part B services are not subject to consolidated billing; therefore, each provider submits a claim to Medicare.
Since the 1990s, the OIG has identified problems with Part B payments for services provided to nursing home residents. An opportunity for fraudulent, excessive, or unnecessary Part B billing exists because nursing homes may not be aware of the services that the providers bill directly to Medicare, and because nursing homes provide access to many beneficiaries and their records.
The OIG review of supplemental payments from CMS has approved Medicaid nursing facility upper payment limit (UPL) supplemental payment programs in several states. In these states, nursing facilities may be eligible for supplemental payments that, when combined with a base payment, may not exceed a reasonable estimate of the amount that Medicare would pay for the services.
Under the UPL supplemental payment programs:
A state may use a variety of financing mechanisms to fund that state’s share of supplemental payments. Medicaid will determine whether payments that states claimed under their Medicaid supplemental payment programs complied with federal and state requirements and describe how those payments were distributed and used.
A review of your cost reports on how your facility utilizes Medicaid UPL payments should be analyzed to determine if your facility is following the regulation.
Last of the financial workplans is the OIG’s review of Skilled Nursing Facilities (SNFs)’ financial responsibility for Medicare Part D enrollee(s) medication during Part A stays and whether SNFs complied with federal requirements for assuming financial responsibility for drugs for Part D enrollees in Part A SNF stays.
Under this review:
Medicare Part A prospective payments to SNFs cover most services, including drugs and biologicals, furnished by an SNF for use in the facility for the care and treatment of enrollees.
Accordingly, Medicare Part D drug plans should not pay for prescription drugs related to posthospital SNF care, because payment for the drugs is included in the prospective payment for a Part A SNF stay. A prior OIG audit found that up to $465.1 million in Part D total cost was improperly paid for drugs for which payment was available under the Part A SNF benefit. That audit also found that some of the drugs administered to Part D enrollees during their Part A SNF stays had been provided to the SNFs by the enrollees or their families, even though the SNFs were financially responsible for providing the drugs. For this audit, the OIG will determine whether SNFs complied with federal requirements for assuming financial responsibility for drugs for Part D enrollees in Part A SNF stays.
When it comes to the OIG’s active workplans that target quality of care and life areas, your organization should
focus on the following: preventable hospitalizations of skilled residents, employee background checks, infection prevention and control, accuracy of documentation of falls and antipsychotics, and staffing and emergency systems.
When it comes to potentially preventable hospitalizations of Medicare-eligible skilled nursing residents, prior CMS studies found that five conditions—pneumonia, congestive heart failure, UTIs, dehydration, and chronic obstructive pulmonary disease/asthma—constituted 78 percent of the long-term care resident transfers to hospitals, according to the OIG October 2022 Workplan item. Additionally, sepsis is often considered a preventable condition when the underlying cause of sepsis can be prevented during the stay.
The OIG will review inpatient hospitalizations of SNF residents with any of these six conditions—pneumonia, congestive heart failure, UTIs, dehydration, chronic obstructive pulmonary disease/asthma, and sepsis—and determine whether the SNF provided services to residents in accordance with their care plans and professional standards of practice (42 CFR § 483.21 and 42 CFR § 483.25).

Care Providers’ Background Checks
The CMS National Background Check Program established the framework for a nationwide program to conduct background checks on a statewide basis on all prospective direct patient access employees of long-term care (LTC) facilities and providers. LTC facilities and providers include skilled nursing and nursing facilities, home health agencies, hospice and personal care providers, LTC hospitals, residential care providers arranging for or providing LTC services, and intermediate care facilities for individuals with intellectual disabilities.
Important to note of this program:
The program’s purpose is to identify efficient, effective, and economical procedures for conducting background checks. The program will be administered by the Centers for CMS, in consultation with the DOJ and the FBI.
OIG work has shown that not all states complied with the National Background Check program for LTC providers. The OIG will determine whether Medicaid beneficiaries in nursing homes in selected states were adequately safeguarded from caregivers with a criminal history of abuse, neglect, exploitation, mistreatment of residents, or misappropriation of resident property, according to federal requirements.
A random review of your facility’s human resources personnel records for background checks should be standard on your compliance audits and cross references with disciplinary actions of employees.
Audit of Nursing Home Infection Prevention and Control Program Deficiencies
The OIG’s nursing home infection prevention and control program deficiency’s objective is to determine whether selected nursing homes have programs for infection prevention and control and emergency preparedness in accordance with federal requirements.
Severe weather events have highlighted the need for and importance of emergency power systems for nursing homes:
In 2023, the OIG announced their review of emergency systems, which is still an active area of focus for 2025. Nursing homes are required to provide an alternate source of energy (usually a generator) to maintain temperatures to protect residents’ health and safety, as well as for food storage, emergency lighting, fire protection, and sewage disposal (if applicable), or to evacuate the residents.
Nursing homes with generators must have them installed in a safe location and are required to perform weekly maintenance checks. During OIG onsite inspections of 154 nursing homes in eight states as part of recent life safety and emergency preparedness audits, of the facilities that had generators, numerous facilities’ generators were more than 30 years old.
The OIG plans to conduct ongoing audits to determine the age of nursing home emergency power systems and whether the systems sustain the wellbeing and safety of their residents. The emergency plans must be reliable and maintain emergency power, food, and water supplies, as well as have alternative facilities for residents who may need to be evacuated per federal regulations.
The PBJ
The OIG also announced in 2023 their audit of staffing hours reported through the Payroll-Based Journal (PBJ). The PBJ data is used by CMS and other stakeholders to monitor nursing home staffing levels, assess quality of care, and identify compliance issues. The OIG is expected to issue audit findings in 2025.
PBJ requirements:
Nursing homes are required to electronically submit complete and accurate direct care staffing information to CMS’s PBJ system on a quarterly basis.
Direct care staff includes nurse and non-nurse staff who, through interpersonal contact with nursing home residents or resident care management, provide care and services to residents to allow them to attain or
maintain the highest practicable physical, mental, and psychosocial wellbeing.
CMS and other stakeholders use the staffing information in the PBJ to:
1 Measure nursing home performance;
2 Better understand the relationship between nursing home staffing levels and the quality of care that nursing homes provide;
3 Identify noncompliance with federal nurse staffing regulations; and
4 Facilitate the development of nursing home staffing measures.
The OIG will audit the nursing staffing hours reported in the PBJ to determine whether the reported hours are accurate and meet regulatory staffing ratios.
Quality of Care
When it comes to quality of care, the OIG has actively focused on clinical areas that are high risk, cause serious injuries, increase cost, and manipulate documentation to avoid regulatory review. The primary clinical areas targeted are falls and antipsychotic medication prevalence, negative impact, and reporting accuracy on the long-term care beneficiaries. The OIG’s active review of these clinical measures was in 2023, while falls were announced in 2024, and they both remain on the active workplan for 2025-2026.
Attending quality of care:
In the Medicare and Medicaid programs, when a nursing home resident experiences a fall, the nursing home is required to report that fall, and the severity of any resulting injury, in a patient assessment.
CMS then uses this information to determine, for each Medicare-certified nursing home, the percentage of residents experiencing falls resulting in major injury. This percentage is posted on CMS’s Care Compare website to give consumers information about the relative performance of each nursing home.
The OIG will assess the accuracy of the patient assessment data used to calculate nursing home fall rates and identify hospitalizations due to falls with major injury among Medicare enrollees receiving nursing home care. In the first study, the OIG assessed the extent to which those falls were reported by nursing homes in patient assessments. This includes the characteristics of residents who did not have their falls reported and the characteristics of nursing homes that did not report falls among their residents.
The OIG will provide additional details about the falls with major injury and hospitalization identified, which could include the amount of time spent in the hospital, the cost of the hospital stays to the Medicare program and enrollees, and outcomes to determine overcoding and billing issues.
Use of Antipsychotic Drugs
The potentially inappropriate use of antipsychotic drugs among nursing home residents remains a concern despite efforts to decrease their use over the last decade. Antipsychotic drugs were developed to treat schizophrenia—a serious mental disorder that is generally diagnosed before the age of 30. These powerful drugs are known to have severe side effects, particularly among elderly individuals with dementia.
In 2008, the Food and Drug Administration issued a boxed warning against the use of all antipsychotic drugs among elderly individuals with dementia because of the increased risk of death.
The OIG raised concerns about the high use of antipsychotic drugs among nursing home residents and, in response, CMS took steps to discourage the use of these drugs by, for example, developing publicly reported quality measures related to the use of antipsychotic drugs among nursing home residents.
More recently, the OIG has raised concerns about the potential falsification of schizophrenia diagnoses to make the use of antipsychotic drugs appear appropriate and avoid federal attention.



TWO HEALTHCARE ITEMS EMPHASIZING THE IMPORTANCE OF STATE LAWS
It is undisputed that complying with both federal and state laws and regulations is required. One of the most significant areas for physician contracts is the noncompetition covenants, which vary from state to state. Another area is cybersecurity.
In June 2025, Texas Governor Abbott signed into law two bills that address the two aforementioned items. Texas SB 1318 refined covenants not to compete for physicians, dentists, physician assistants, and nurses (hereinafter “medical professionals”). Separately, Texas SB 2610 establishes a limited cybersecurity damages limit for businesses with fewer than 250 employees. Both Ohio and Utah implemented similar laws in 2018 and 2021, respectively. The remainder of this article provides additional details into these two recent Texas law developments.
Texas Two-Step
Non-compete covenants are commonly included in physician contracts, sales of medical practices, and other employment and independent contractor agreements involving medical professionals. On June 20, 2025, TX SB 1318 was signed into law and amends Texas Business and Commerce Code §§ 15.50, 15.51. Notably, it only applies to non-compete covenants entered into or renewed on or after September 1, 2025. Stated another way, any non-compete covenant entered into or renewed prior to September 1, 2025 by medical professionals has the existing law at that time applied to it.
Beginning September 1, 2025, medical professionals need to be aware of covenants restricting competition, which include the following items:
Expire no later than one year from the termination of their employment or contract;
Limit the geographical area subject to the covenant to no more than a five-mile radius from the location where the medical professional primarily practiced before termination;
Provide for a buyout of the covenant by the medical professional in an amount that is not greater than their total annual salary; and
Contain terms and conditions that are clearly and conspicuously stated in writing.
Moreover, if a Texas Medical Board licensed physician is involuntarily discharged without “good cause,” which is defined in §15.50, then the non-compete provision is void and unenforceable.
Cybersecurity is an equally pressing issue for medical professionals. TX SB 2610 adds Texas Business and Commerce Code Chapter 542 (“cybersecurity program”) and becomes effective September 1, 2025.
Here are the key takeaways
Applies to businesses with less than 250 employees and own or license computerized data that includes sensitive personal information (see §521.002 for definition);
Exemplary damages are prohibited; Cybersecurity programs must be implemented that protect personal identifying information (PII) and sensitive personal information through implementing adequate technical, administrative, and physical safeguards, which vary depending on the following

tranches: (1) 20 or fewer employees; (2) 21-99 employees; and (3) 100–250 employees; and
Recognized cybersecurity frameworks include the National Institute of Standards and Technology (NIST), Federal Risk and Authorization Management Program’s (FedRAMP) Security Assessment Framework; and HIPAA and the HITECH Act.
Importantly, businesses with more than 251 employees are not affected by this law. Additionally, class actions can still be filed, and compliance with the vast swath of laws tied to cybersecurity programs in §542.004(b)(1),(2), including HIPAA, is not optional.
Conclusion
Staying abreast of the evolving state legal and regulatory landscape is equally as important as staying abreast of the dynamic federal landscape. It is recommended that the aforementioned laws be read and incorporated into policies and procedures, as well as contracts when appropriate.
Rachel V. Rose, JD, MBA, advises clients on compliance, transactions, government administrative actions, and litigation involving healthcare, cybersecurity, corporate, and securities law, as well as False Claims Act and Dodd-Frank whistleblower cases. She also teaches bioethics at Baylor College of Medicine in Houston. Rachel can be reached through her website: www.rvrose.com
4-STEP APPROACH TO OVERCOMING PATIENT ACCESS CHALLENGES
CENTRALIZATION

We recently explored the challenges that healthcare organizations face with patient access, which create significant operational issues that impact revenue and the patient financial experience. To eliminate fragmented workflows and streamline processes, healthcare organizations can take practical steps to centralize patient access and implement an Integrated Financial Clearance (IFC) model.
Centralization, which involves the consolidation of frontend administrative functions, such as scheduling, insurance verification, prior authorization, and patient estimates, into a single, standardized system or team within a healthcare organization, can help address these challenges. Centralized patient access is designed to improve efficiency, ensure
data accuracy, reduce front-end claim denials, enhance the patient experience, and lower the cost of collection.
A phased, stepwise approach to centralization can help healthcare organizations avoid disruption, address workforce gaps, and build a scalable, technology-enabled model for success.
Step 1
Standardize Patient Access Workflows
The first step in centralization is to standardize patient access workflows. This involves identifying and documenting the ideal processes and technologies, then
eliminating bottlenecks that can impede efficiency. For example, many healthcare organizations still rely on outdated systems or manual processes for eligibility verification and prior authorization, creating unnecessary delays and errors. Standardizing these processes, with the support of modern technology, allows teams to work more effectively and reduces the chances of claim denials due to errors.
Step 2
Implement Phased Centralization
Once ideal state workflows are standardized, the next step is to introduce centralization in phases. This approach minimizes disruption and allows for the effective allocation of resources. During this phase, healthcare organizations must evaluate their staffing strategy and identify gaps to centralize financial clearance operations. A hybrid model of in-house and global support, particularly for manual tasks like prior authorization, can provide the scalability needed to meet demand while ensuring compliance with regulations.
Step 3
Optimize Technology Solutions for Automation and Analytics
Once the ideal delivery model has been achieved, the focus shifts to optimizing existing processes and technology solutions. Technology solutions should leverage automation to streamline operations, reduce manual tasks, and enhance the patient experience. Automation tools for eligibility checks, prior authorization, and claims management can reduce errors and improve turnaround times. Additionally, using AI-powered analytics allows organizations to measure outcomes such as reduced authorization delays, proactively identify trends, track performance, and continuously improve patient access workflows.
Step 4
Measure Success & Drive Accountability
Centralization requires continuous monitoring to ensure it is delivering the expected results. Key performance indicators (KPIs) should be established to track progress and drive accountability, such as reduced authorization turnaround time, improved clean claims rate, and enhanced patient satisfaction. Regular performance evaluations will help identify areas for improvement and ensure that the centralization strategy remains aligned with organizational goals.
Ryan Chapin, Executive Director of Strategic Solutions at AGS Health, assists with strategic growth initiatives for the company’s Patient Access and Patient Financial Services business units. He possesses more than 8 years of experience in professional and managed services with expertise in delivering clients transformational engagements focused on improving financial and operational metrics, and the patient experience. Leveraging his background in Revenue Cycle Consulting, Ryan brings a true consultative approach to how AGS conducts business with their customers.
Ryan
Chapin and Hari Shankar
HariShankar Veeraji Baskaran, Associate Director for the Patient Access and Patient Financial Service business units at AGS Health, plays a key role in driving market awareness for Sales and Customer Success, expanding service and product offerings. As a subject matter expert, Hari supports strategic deal solutioning while championing digitization, analytics, and automation to improve efficiency and financial outcomes in the healthcare revenue cycle. With more than 20 years of experience in accounts receivable (A/R) revenue cycle management (RCM), Hari has a proven track record of managing large client portfolios and leading high-performing, geographically dispersed teams. His expertise in service line adherence and financial performance has helped organizations achieve sustainable revenue growth and operational excellence.
https://www.agshealth.com/
adVanced life SupporT leVel 2 (alS2) 2025 updaTeS

Advanced Life Support Level 2 (ALS2) is the highest level of ground ambulance service defined by the Centers for Medicare and Medicaid Services (CMS).
ALS2 includes advanced procedures beyond ALS1, such as:
Administration of three or more medications (IV, IM, or SQ)
Manual defibrillation/cardioversion
Endotracheal intubation
Central venous line placement
Chest decompression, intraosseous line, or transtracheal jet ventilation
HCPCS Code: A0433
The HCPCS code for ALS2 remains A0433, which is billed per transport. CMS finalized changes in the Calendar Year (CY) 2025 Physician Fee Schedule (PFS) regarding the definition of Advanced Life Support, Level 2 (ALS2) procedures for ambulance services.
Previously, the definition of ALS2 for ambulance services included certain medications and complex medical procedures. The new guidelines expand this definition to include prehospital blood transfusions (PHBTs).
This means that ground ambulance transport involving PHBTs, such as the administration of low titer O+ or O- whole blood, packed red blood cells, or plasma, can now qualify as an ALS2-level transport.
While this expands the services covered under ALS2, it’s important to note that CMS did not propose additional funding for these services, as most transports involving whole blood were already reimbursed at the ALS2 level or higher due to the presence of other ALS2 procedures.
2025 Changes to Know
Updated Definition of Medication Administration: CMS clarified that saline or glucose solutions alone no longer count toward the three-medication minimum for ALS2 unless accompanied by therapeutic agents.

Medications must have therapeutic intent, not just serve as vehicles.
Expanded List of Qualifying Procedures: New guidance includes continuous positive airway pressure (CPAP) and automatic chest compression devices as acceptable ALS2-level procedures if medically necessary and properly documented.
Emphasis on Documentation: Claims must include clear narratives and procedure timestamps. ALS2 services without strong documentation are now more frequently denied during audits.
Bundled Services Clarification: ALS2 continues to bundle services like oxygen, EKG monitoring, and routine airway management. Coders should not bill separately for these elements if ALS2 is billed.
Common Errors to Avoid
Reporting A0433 without confirming three qualifying meds or at least one ALS2-level procedure.
Failing to document the name, dose, and route of each medication.
Missing certification of medical necessity.
Billing ALS2 when ALS1 would suffice (risk of overpayment and audit flags).
Tips for Accurate ALS2 Coding
Review run sheets closely for qualifying interventions.
Verify protocol to ensure treatments meet ALS2 criteria. Coordinate with EMS teams to improve documentation compliance.
Use real-time documentation tools when available.
Conclusion
The new ALS2 rules highlight the need for clear, defensible documentation and a strong understanding of qualifying criteria. Coders, billers, and EMS professionals must work together to ensure proper reporting and reimbursement.
Janine Mothershed, CPC, CPC-I, is the founder and CEO of Coding Clarified, an innovative online medical coding school committed to transforming lives through flexible, high-quality career training. A Certified Professional Coder (CPC) and licensed AAPC instructor, Janine brings over a decade of experience in healthcare administration, medical coding, and workforce development. Codingclarified.com
NEW MR SAFETY CODES PROVIDE ADDITIONAL REIMBURSEMENT FOR RADIOLOGISTS
Anew subsection of the CPT code book has been established for reporting six new codes describing MR safety services, including implant or foreign body evaluation, safety consultation, electronics preparation, and implant positioning or immobilization. The new subsection, Magnetic Resonance (MR) Safety Implant/Foreign Body Procedures of the Radiology/Diagnostic Radiology (Diagnostic Imaging), contains the codes along with the guidelines for reporting them.
These new codes describe the work that takes place prior to an MRI study, including the proper assessment, consultation, and medical physics customization for patients who have an implant, device, or foreign body. Examples include cochlear implants, artificial valves, deep vagal stimulators, pacemakers, apnea devices, etc. Routine submission of these codes for every patient with an implant, device, or foreign body undergoing MR imaging is not expected.
Documentation & Billing Considerations
Procedure codes 76014 and 76015 are Technical Component (TC) codes that reflect the work of an MRI technologist and/ or a medical physicist. They do not include any physician work value, but they would be available in the imaging center using global billing. The others contain a professional component that would be available for the radiologist to bill in either a hospital or imaging center setting. Note that the physician performing the safety assessment does not have to be the same as the physician interpreting the MRI exam. In some cases, the safety assessment may determine that the MRI scan should not be performed; however, the appropriate safety codes may still be billed with no related imaging billing.
Each of the CPT code descriptions specifies that it includes a written report. The safety assessment documentation should be separate from the interpretation of the imaging examination, as the assessment may be conducted days or weeks before the actual MRI scan.
The Medicare Physician Fee Schedule (MPFS) for 2025 established fees for the new codes are shown in Table 1.
Note: G = Global; PC = Professional Component.
Medicare Fee represents the national level using the CF of $32.3465 in effect as of 1/1/2025.
As time-based codes, 76014 and 76015 require that at least half of the stated time is completed to qualify for billing and the documentation for them should specify the exact time expended. They are performed by the non-physician staff, who normally are not responsible for creating such documentation, and so each facility will have to establish its own process to capture this work. The documentation should include the ordering physician’s information (name and NPI number) and the reason for the exam for billing purposes.
Physicians performing the assessment and developing the imaging customization for codes 76016–76019 should adequately describe the steps taken and the resulting parameters for the exam.
The report for 76016, which can occur prior to the time of the exam, should include:
A risk-benefit analysis
Evaluation for conformance with the implanted device safety instructions
Consideration of alternative diagnostic tests when MRI is deemed to be inappropriate
The remaining codes (76017–76019) represent real-time interaction while the patient is present for the exam.
Documentation relevant to the particular code should include:
The conditions present that would necessitate an adjustment of the imaging protocol
A description of the adjustments made
Any limitations of the exam as a result of the protocol adjustments
A description of the real-time adjustments to the programming of the patient’s device
A description of the steps taken to immobilize the patient or position a device to make it safer for the patient
Discussion with the patient prior to the exam,

FREE WEBINARS & CEUS! MORE
Specialty Outreach and Behavioral Health Coding
Presenter: Mariellen Mezzacappa CPC, CRC, CPMA
Time: 54.03 minutes
Many claims are denied because they do have the correct code or modifier, which are prone to change and vary by payor. This webinar delves into common specialty outreach and behavioral health codes and modifiers to help you distinguish the appropriate code(s) to avoid denials.
CEUs are available for all of the following associations at no additional cost: AAPC, AHIMA, ARHCP, PMI, PAHCS, PHIA, POMAA, MAB, MED-C, HBMA, NEBA, PAHCOM, AHCAE, PMBA
www.billing-coding.com/ceus
including obtaining
Reasons for discontinuation it
Recommendations experience
A separate order for the order for the MR to determine if the When the TC-only codes technologist, no rendering NPI number is to be billing, the TC-modifier
Summary
The TC-only codes the hospital or the associated with intended to be billed medical device or might be at risk nature of the need a projected level if one patient per the average PC reimbursement radiologists for performing the national Medicare
The establishment reimbursement for standard patient as of January 1, 2025, commercial payor. value of the work billing these new
Erin Stephens, at Healthcare Partners (HAP) analytics, and radiology practices, practices. www.hapusa.com

Medical Coding bundle - online Series


Introduction to Medical Coding
Introduction to ICD-10-CM Coding
Introduction to CPT Coding
Introduction to E/M Coding
Medical Terminology
Principles of Coding


PMI Instructor Jan Hailey will take you on an interactive walk through medical coding for outpatient services.

The CMC course includes a physical course manual, nine instructor-led live online classes, bonus study sessions, instructor one-on-one support, and the CMC certification exam.


Review examples from each ICD-10 chapter and body system in CPT, demonstrate the step-by-step process to identify correct codes, and review challenging concepts.
The Certified Medical Coder live online class series begins on September 10.
Enroll now to begin the 6 foundation classes and bonus training videos. 35 bonus training videos View Medical Coding Bundle free online assessment start start here here here




monThly SpoTlighT on fraud, WaSTe, and abuSe
The following cases highlight fraud, waste, and abuse (FWA) and serve as a reminder to uphold high ethical standards when providing patient care and services.
CEO of Healthcare Software Company
Convicted of $1 Billion Fraud Conspiracy
A federal jury convicted the CEO of Power Mobility Doctor Rx, LLC (DMERx) for his role in operating a platform that generated false doctors’ orders to defraud Medicare and other federal healthcare benefit programs of more than $1 billion.
According to court documents and evidence presented at trial, a 79-year-old man of Maricopa County, Arizona, and his co-conspirators targeted hundreds of thousands of Medicare beneficiaries who provided their personally identifiable information and agreed to accept medically unnecessary orthotic braces, pain creams, and other items through misleading mailers, television advertisements, and calls from offshore call centers. He and his co-conspirators owned, controlled, and operated DMERx, an internet-based platform that generated false and fraudulent doctors’ orders for these items.
As part of the scheme, he connected pharmacies, durable medical equipment (DME) suppliers, and marketers with telemedicine companies that would accept illegal kickbacks and bribes in exchange for signed doctors’ orders transmitted using the DMERx platform. He and his
co-conspirators received payments for coordinating these illegal kickback transactions and referring the completed doctors’ orders to the DME suppliers, pharmacies, and telemarketers that paid kickbacks and bribes for the orders.
The fraudulent doctors’ orders generated by DMERx falsely represented that a doctor had examined and treated the Medicare beneficiaries when in fact purported telemedicine companies paid doctors to sign the orders without regard to medical necessity, based only on a brief telephone call with the beneficiary or no interaction with the beneficiary at all. The DME suppliers and pharmacies that paid illegal kickbacks in exchange for these doctors’ orders billed Medicare and other insurers more than $1 billion. Medicare and the insurers paid more than $360 million based on these claims. According to evidence presented at trial, he and his co-conspirators concealed the scheme through sham contracts and by eliminating from doctors’ orders what one co-conspirator described as “dangerous words” that might cause Medicare to audit the scheme’s DME suppliers.
He was convicted of conspiracy to commit healthcare fraud and wire fraud, three counts of healthcare fraud, conspiracy to pay and receive healthcare kickbacks, and conspiracy to defraud the United States and make false statements in connection with healthcare matters. The man faces a maximum penalty of 20 years in prison for the conspiracy
to commit healthcare fraud and wire fraud conviction, 10 years for each healthcare fraud conviction, five years for the conspiracy to pay and receive healthcare kickbacks conviction, and five years for the conspiracy to defraud the United States and make false statements in connection with healthcare matters conviction. A sentencing hearing will be scheduled later. A federal district court judge will determine any sentence after considering the U.S. Sentencing Guidelines and other statutory factors.
Source: CEO of Health Care Software Company Convicted of $1 Billion Fraud Conspiracy (2025, June 3). www.justice.gov
Jorden Borders Guilty on All Counts in Child Torture and Medicaid Fraud Case
A Minnesota District Court Judge found a woman guilty of all 11 charges: one count of attempted murder, three counts of child torture, three counts of stalking, and four counts of theft by false representation.
As proven at trial, the woman physically, verbally, and emotionally abused her three minor children for more than five years. Her acts included, but were not limited to, engaging in medical child abuse by performing specific acts against her children that led to them presenting with false medical conditions to their medical providers. This included the woman forcibly withdrawing blood from her then nine-year old child prior to his doctor’s visits with a syringe, through a PICC line, or through a central line. She then presented the child at doctor’s visits and hospitals, where he was observed as having dangerously low hemoglobin levels. She also self-diagnosed her children with other diseases, including osteogenesis imperfecta (brittle bone disease) and forced her children to wear boots, casts, and neck braces even though they did not have any identified fractures or diagnosed injuries. One child was determined to have been in a cast for nearly two years and two months of his life despite having only two confirmed injuries from medical professionals. Another child stated that the mother would instruct the child to vomit at the doctor even though he didn’t need to, and to cough as if he had asthma, at which time asthma medication was prescribed. The defendant also forced one of the children to wear unprescribed hearing amplifiers, which led to the child developing a boil on the back of her ear.
The children also all testified that she hit the children with charging cords, belts, and spoons, made them stand outside in the cold without clothing until their bodies felt like burning, withheld food, and regularly threatened to kill them. At times, the threats to kill were accompanied with knives and guns. Social media evidence from her accounts indicated that she regularly discussed “end of life” decisions for one of the children with others, including at one point falsely stating that one of her children was on a ventilator and that they wouldn’t know the cause of his death until an autopsy.
The woman also met with Crow Wing County Community Services to request funding to care for one of the children through the Minnesota medical assistance (Medicaid) program. She provided false information about the child’s medical condition. As a result of this false information, the child was approved to receive personal care assistant (PCA) services. She claimed to provide these PCA services and received over $18,000. Trial witnesses testified that if they had known about her actions and the false information she provided, PCA services would not have been authorized. The investigation also found that she had been nominated to receive several gifts and money from non-profit foundations for her children, with these gifts based on the false information she provided about her children’s medical conditions.
The judge ordered Borders held without bail and set a sentencing date of August 7. Prior to sentencing, the court will issue an order on aggravating factors. Borders’ parental rights to the three children had previously been terminated. Attorney General Ellison’s Medicaid Fraud Control Unit works to uncover, investigate, and prosecute individuals or organizations that steal from Medicaid and that exploit, neglect, or abuse vulnerable victims.
Source: Jorden Borders Guilty On All Counts In Child Torture And Medicaid Fraud Case (2025, June 6). www.ag.state.mn.us
Sonal Patel, BA, CPMA, CPC, CMC, ICDCM, is CEO and Principal Strategist of SP Collaborative, LLC. Sonal has over 13 years of experience understanding the art of business medicine as a nationally recognized thought-leader, speaker, author, creator, and consultant to elevate coding compliance education for the business of medicine. www.spcollaborative.net

iS iT Time To hire a coder? a guide for Small pracTiceS
Small practices, typically made up of one to five providers, often find themselves needing to be fiscally conservative and operationally adaptable. But when it comes to coding and billing, those traits can sometimes lead to short-term solutions that create long-term problems.
Coding isn’t just about selecting the right CPT or ICD-10 code, it’s about understanding regulatory requirements, supporting documentation, and preserving the integrity of the revenue cycle. As a result, many practices face the same important question: Is it time to bring a coder in-house?
Let’s break that down. Because the answer doesn’t rest solely on practice size, but on the services provided, the complexity of coding requirements, and the risks tied to getting it wrong.
Pros and Cons of Hiring a Coder In-House
As with any business decision, the pros and cons should be considered when adding an additional operational expense. For small practices navigating limited resources and increasing documentation demands, understanding both the benefits and the limitations of bringing coding in-house is essential.
Pros
Real-Time Support: An in-house coder can offer immediate feedback, help clarify documentation before it leads to denials, and promote accurate claim submission from the outset.
Improved Internal Communication: Coders embedded in the practice better understand team workflows, clinical terminology, and operational goals, enhancing collaboration.
Proactive Compliance Monitoring: Having someone on site allows practices to identify trends and resolve issues before they trigger audits or revenue loss.
Cons
Cost Considerations: Hiring a full-time coder brings salary and training expenses that can be difficult for a lean-budget practice to justify.
Recruitment Challenges: Especially in underserved regions, it can be difficult to find qualified coders who understand both the specialty and the payor environment.
Scope of Expertise: A single coder may not be equipped to handle multiple service lines and specialties without additional training and support.
Regardless of who is doing the coding, the responsibility for submitted claims ultimately falls to the rendering provider. That’s why physicians and advanced practice providers must have a fundamental understanding of coding principles and documentation standards.
You cannot code your way out of poor documentation. And no matter how skilled your coder is, they cannot assume clinical intent. The provider’s documentation must clearly support the services billed. In the event of an overpayment or audit, it’s the provider’s name on the letter.
Therefore, an emphasis should be placed on responsibility and awareness. Provider engagement in the coding process is essential, because when that engagement is present, outcomes measurably improve.
Combination Role
For many small practices, combining the roles of coder and biller into one position feels like a natural fit. It can streamline workflows, save on staffing costs, and improve communication across the billing cycle. However, this arrangement comes with its own set of considerations, and practices must weigh the risks against the rewards before moving forward.
Rewards of a dual role:
It can be a cost-effective staffing solution, especially for practices operating with tight budgets.
Communication and workflow may be more seamless when one person handles both the coding and billing components.
A single point of responsibility can lead to quicker claims processing and fewer handoffs or delays.
With the right knowledge base, the dual role can foster a deeper understanding of the entire revenue cycle process.
Risks of a dual role:
Without separation of duties, errors in coding may go undetected, particularly if no secondary review process is in place.
The lack of peer review increases the risk of noncompliance and missed audit vulnerabilities.
One person juggling both tasks can lead to burnout or decreased focus on the details that each function requires.
There may be limited time or resources for continuing education, which is critical in both disciplines.
Success in a combination role hinges on the skill, training, and accountability of the individual filling it. Practices choosing this route must ensure that proper safeguards— such as internal reviews and ongoing education—are in place to support accuracy and mitigate risk.
Is a Certified Coder Necessary?
While certification can be a helpful benchmark, it should not be the sole measure of a coder’s capability. Certification indicates that a coder has demonstrated general knowledge and is prepared to meet baseline industry expectations, but it does not always reflect the depth of applied understanding needed in a real-world, specialty-specific setting.
What truly matters in a small practice is a coder’s ability to interpret, apply, and adapt coding guidelines to your services, documentation styles, and payor requirements. The difference lies in how well they translate rules into practice,
not just whether they passed an exam.
Whether certified or not, a strong coder brings working knowledge, critical thinking, and an ongoing commitment to learning—because coding isn’t static. Rules change, payor policies shift, and complexity grows. The right coder is the one who keeps pace and brings practical expertise to the table.
Investing in applied knowledge, supported by continuing education and specialty-specific training, is far more impactful than focusing on credentials alone.
Is It Time to Make Room for a Coder?
Hiring a coder is not just a matter of how many patients you see; it’s about whether the complexity of your services demands a higher level of oversight, accuracy, and coding support. Practices should begin by evaluating their current pain points and the nature of the services they provide. If coding questions are common, denials are increasing, or documentation reviews are highlighting gaps, those are signals to reassess your current structure.
Ask yourself the following:
Are you performing procedures in-office that require modifier usage or global period tracking?
Are you billing for care management services such as CCM or TCM, or regularly reporting higher-level E/M?
Are you involved in shared or split services, requiring careful attribution and documentation alignment?
Do you routinely submit claims with modifiers, add-on codes, or specialty-specific requirements?
If these scenarios are part of your routine, the demand on your coding process is high.
That level of complexity is a strong indicator that it may be time to bring a coder in-house—or at minimum, secure dedicated coding expertise. The decision should be based on the services you provide, the rules those services trigger, and the risk that comes with getting them wrong.
Ultimately, it’s about supporting compliance, protecting reimbursement, and setting up your practice to grow with confidence and clarity.
The business side of healthcare doesn’t allow for guesswork. Accuracy, documentation integrity, and timely reimbursement all depend on a clear strategy for coding and billing.
Small practices can succeed with internal coders, external support, or a hybrid model—but that decision must be intentional. At the end of the day, a coder can guide the process, but the provider always remains responsible.
Shannon Deconda is the Director of Coding and a DoctorsManagement partner. She is also the founder and president of NAMAS (National Alliance of Medical Accreditation Specialists), an organization that provides education for medical documentation auditing, compliance, and exam preparation for the AAPC’s national auditing credential CPMA (Certified Professional Medical Auditor). Shannon brings more than 16 years of multi-specialty auditing and coding experience to DoctorsManagement clients. Using her coding and auditing expertise, she helps practices optimize business processes and maximize reimbursements. Shannon identifies missed revenue opportunities for clients, as well as coding habits that may put the practice at risk. Shannon then trains practice providers and staff based on their specific areas of need, empowering them with techniques that help them receive full and timely reimbursement for all the services provided to patients. Her clients often realize improvements in cash flow as a result of the insight and education they receive. https://namas.co/

MASTERING MEDICAL CODING AND BILLING FOR ALZHEIMER’S DISEASE: GUIDELINES
AND TIPS
Alzheimer’s disease, a progressive neurodegenerative disorder, is one of the leading causes of disability and dependence among older adults. Accurate coding and billing for Alzheimer’s disease is crucial not only for proper reimbursement but also for clinical documentation and care coordination. This article provides essential guidelines and expert tips for correctly coding and billing services related to Alzheimer’s disease in compliance with ICD-10-CM and CMS regulations.
Understanding Alzheimer’s Disease in Medical Coding
Alzheimer’s disease is classified under ICD-10-CM category G30, which includes:
G30.0 – Alzheimer’s disease with early onset
G30.1 – Alzheimer’s disease with late onset
G30.8 – Other Alzheimer’s disease
G30.9 – Alzheimer’s disease, unspecified
When coding Alzheimer’s, it’s important to determine the onset (early vs. late), the presence of behavioral disturbances,
Coding Guidelines: Primary vs. Secondary Diagnosis
Alzheimer’s disease is often listed as a primary diagnosis when it is the main reason for a patient’s encounter or when it is being directly managed or evaluated.
Primary vs. secondary guidelines:
If a patient presents for complications or symptoms related to Alzheimer’s (e.g., wandering, aggression, cognitive decline), Alzheimer’s should typically be coded as the primary diagnosis.
For encounters focused on comorbidities (e.g., diabetes, hypertension), Alzheimer’s may be reported as a secondary diagnosis, provided it affects patient care, management, or treatment.
Use of Additional Codes
In most cases, Alzheimer’s disease should be reported in conjunction with additional codes to capture:
Behavioral disturbances
• F02.81 – Dementia in other diseases classified elsewhere with behavioral disturbance
• F02.80 – Without behavioral disturbance
Cognitive and mood symptoms
• R41.81 – Age-related cognitive decline
• F03.90 – Unspecified dementia without behavioral disturbance
• F03.91 – Unspecified dementia with behavioral disturbance
Delirium or psychosis, if present
Tip
Always verify documentation for behavioral and psychological symptoms to code them accurately. Query providers when necessary.
A new blood test, the Lumipulse G pTau217/ß-Amyloid 1-42 Plasma Ratio, has been cleared by the FDA for the early detection of Alzheimer’s disease in adults aged 55 and older who exhibit signs and symptoms. This test detects amyloid plaques, which are associated with Alzheimer’s disease, and provides a less invasive and more accessible alternative to existing diagnostic methods.
The HCPCS code for Lumipulse G pTau217/ß-Amyloid 1-42 Plasma Ratio is not a standard HCPCS code. Instead, it’s a Proprietary Laboratory Analyses (PLA) code, specifically, which is CPT code 0358U.
Billing for Related Services
When billing for Alzheimer’s-related care, ensure documentation supports services, such as:
Evaluation and Management (E/M) services, including cognitive assessment and care planning
Neuropsychological testing
Behavioral health interventions
Caregiver counseling and coordination
CMS G codes (used in Medicare billing), such as G0505 (cognitive assessment and care plan services), may apply.
Tip: Confirm that the patient’s insurance covers the services and that medical necessity is clearly documented.
Avoid the following errors when coding for Alzheimer’s disease:
Using unspecified codes (e.g., G30.9) when specifics are available
Failing to code for behavioral disturbances
Omitting coexisting conditions or symptoms that impact care
Lack of linkage between Alzheimer’s and dementiarelated manifestations
Compliance and Ethical Considerations
Coding for Alzheimer’s should reflect:
Accurate Clinical Documentation: Confirm diagnosis in the patient record by a qualified provider.
Medical Necessity: Justify each service and test related to Alzheimer’s care.
Regular Updates: Stay current with ICD-10-CM guidelines and payor policies, especially for Medicare Advantage plans.
Proper coding and billing of Alzheimer’s disease requires a nuanced understanding of its clinical presentations, coding conventions, and payor requirements. By adhering to guidelines and enhancing collaboration with providers, medical coders can ensure that documentation reflects the complexity of care and supports appropriate reimbursement.
Janine Mothershed is the founder and CEO of Coding
Clarified, an innovative online medical coding school committed to transforming lives through flexible, high-quality career training. A Certified Professional Coder (CPC) and licensed AAPC instructor, Janine brings over a decade of experience in healthcare administration, medical coding, and workforce development. codingclarified.com

