CoQ10 Application for Improving Human Health / The Role of Gut Microbiome in Excess Body Weight / Consumer Supplement Survey: Omega-3 Users / The Omega Industry Expert Panel / Formulating Product for Cellulite Reduction and Skin Firmness


CoQ10 Application for Improving Human Health / The Role of Gut Microbiome in Excess Body Weight / Consumer Supplement Survey: Omega-3 Users / The Omega Industry Expert Panel / Formulating Product for Cellulite Reduction and Skin Firmness

Dear readers,
We are pleased to present issue No.15 March/April of B2B Nutramedic&Cosmetics.
In this issue, we focus on the primary role of the gut microbiome in weight management and combating inflammatory disease. This month, we highlight the revolutionizing potential of MicroActive® nutrients in supplement technology and examine increased bioavailability and health impact of CoQ10. Omega-3 continues in the limelight with coverage of the ITC 2024 Consumer Supplement Survey, expert insight, and innovations in purification procedures to ensure highest purity and strength. On the beauty front, we explore a biotechnological revolution: exosomes in cosmetics, which are transforming skincare innovation. Additionally, L'Oréal Groupe brings revolutionary innovations in consumer skin intelligence. Our special formulation feature this month is on tackling cellulite and improving skin firmness, offering solutions that act from the inside out. Looking ahead, we’re thrilled to announce our media partnership with Vitafoods Europe 2025 in vibrant Barcelona. This collaboration underscores our commitment to connecting industry professionals with unparalleled opportunities for inspiration, collaboration, and growth. Don’t miss this key event, a must-attend for anyone passionate about the future of health and nutrition. We hope these stories evoke wonder, inspire imagination, and bring new potential to health and beauty. Thank you for being a member of our community. Enjoy your reading!

Daria Šurić, MPharm, univ.spec.pharm.
EDITOR-IN-CHIEF
B2B Nutramedic&Cosmetics Magazine



The Role of Gut Microbiome in Excess Body Weight
A Promising Approach to Mitigate Gut Inflammatory Diseases
Why Choosing MicroActive® Nutrients for Food Supplement Development Always Pays Off?
OMEGA-3 USERS: Highlights From The ITC 2024 Consumer Supplement Survey
Bimonthly digital magazine for industry professionals in health, nutrition and cosmetics sector
Bimonthly digital magazine for industry professionals in health, nutrition and cosmetics sector

The Omega Industry Expert Panel
Ingredients and raw materials / Contract manufacturing Equipment & Packaging / Services / Industry events
www.nmc-magazine.com
Ingredients and raw materials / Contract manufacturing Equipment & Packaging / Services / Industry events info@nmc-magazine.com
info@nmc-magazine.com
Publisher: Darmell d.o.o.
Publisher: Darmell Ltd.
Cvjetna cesta 11, 10000 Zagreb, Hrvatska Mob: + 385 91 68 12 444 darmell@protonmail.com www.dar-mell.com
Cvjetna cesta 11, 10000 Zagreb, Croatia Mob: + 385 91 68 12 444 darmell@protonmail.com www.dar-mell.com
Supported by www.inpharma.hr

Innovative Refinement Process for Healthy and Pure Omega-3 Fatty Acids
The publisher does not assume responsibility for the opinions and data that the authors present in the magazine, as well as for the data and materials provided by companies for publication in texts and advertisements. It is not allowed to reuse any part of the content without the prior consent of the publisher.
The publisher does not assume responsibility for the opinions and data that the authors present in the magazine, as well as for the data and materials provided by companies for publication in texts and advertisements. It is not allowed to reuse any part of the content without the prior consent of the publisher.

CoQ10 Application for Improving Human Health and Bioavailability Challenges



33 The Effect of Coenzyme Q10 and Vitamin E on Metabolic and Hormonal Profiles in Women with PCOS
34 Tesnor® Demonstrates Support for Sexual Function in Aging Men
35 Levagen®+ Demonstrates Support to Ease Menstrual Discomfort
36
Tres, dos, uno – the Countdown is On for Vitafoods Europe 2025 in Barcelona













40
FORMULATING PRODUCT
GUIDE: Cellulite Reduction and Skin Firmness from the Inside Out
42 The Exosomes in Cosmetics: A True Biotechnological Revolution Bringing the Future into the Present
44 L'Oréal Groupe Unveils Revolution in Consumer Skin Intelligence
45
MEDICINAL PLANTS PHOTO HERBARIUM: Hawthorn (Crataegusoxyacantha)








Over the past decade, the gut microbiome has been recognized as a key factor in the development and progression of obesity and related diseases, making it the subject of intense research7,8. Research indicates that individuals with diet-induced obesity have significant changes in their gut microbiome, including decreased bacterial diversity and an increased ability to extract energy from food, which may contribute to metabolic disorders.
AUTHORS:
Sara Kralj, MSc, Master in Nutrition & Ira Renko, MSc, Master in Molecular biotechnology
According to the International Classification of Diseases 11 (ICD-11), obesity is defined as a chronic and complex disease characterized by excessive fat accumulation that may harm health. In most cases, it is a multifactorial disease caused by an environment that promotes weight gain, psychosocial factors, and genetic variations.
In a subset of patients, specific primary causes can be identified (medications, diseases, immobility, medical procedures, monogenic diseases/genetic syndromes).
Additionally, obesity is associated with a range of health conditions and diseases, including cardiovascular diseases, type 2 diabetes (T2DM), obstructive sleep apnea, and osteoarthritis, emphasizing its sig-
nificant impact on the development of various disorders, making it one of the leading causes of mortality worldwide1.
Body mass index (BMI) serves as a surrogate indicator of body fat and is calculated as the ratio of body weight (kg) to the square of height (m²). BMI categories for defining obesity vary depending on age and sex in infants, children, and adolescents2 According to the World Health Organization (WHO), overweight and obesity are defined as excessive or abnormal fat accumulation that may pose a risk to health. BMI is less reliable in individuals with well-developed lean mass, where a high BMI does not necessarily indicate increased fat mass, and should therefore be considered a rough guideline3,4.

Since 1975, global obesity rates have nearly tripled, making it one of the most significant public health issues in today's society5,4. Previously, obesity was considered a problem mainly affecting high-income countries. However, overweight and obesity have become global concerns, including in middleand low-income countries, particularly in urban areas2,5
The WHO highlights the growing issue of obesity affecting an increasing number of children and spreading across all continents. WHO data from 2016 recorded more than 340 million children and adolescents aged 5 to 19 as overweight or obese. The prevalence of overweight and obesity in this age group has increased dramatically from 4% in 1975 to over 18% in 2016. The rise is similar among both boys and girls, with 18% of girls and 19% of boys classified as overweight in 20165
The etiology of obesity is complex. Obesity is influenced by factors that cannot be controlled, such as genetic, biological, or endocrine factors, as well as factors that can be influenced, such as environmental and social factors, along with other factors that may contribute to the development of obesity6,4 Over the past decade, the gut microbiome has been recognized as a key factor in the development and progression of obesity and related diseases, making it the subject of intense research7,8. The gut microbiota plays a crucial role in the maturation of innate immunity during early life, while simultaneously recognizing and modulating numerous environmental signals and influencing various physiological processes. It acts as an intermediary between the host and external factors, potentially impacting human health. Changes in the composition of beneficial bacteria can have significant consequences, including the promotion of certain aspects of disease development. The microbiota can be influenced by various factors, such as diet, diseases, medication use, and infections9
A healthy gut microbiome is characterized by high diversity and balance, which are essential for maintaining immune homeostasis and resistance to pathogens. Additionally, the microbiome establishes a synergistic relationship with the host, influencing nutrient absorption, energy regulation, and protection against pathogens10-12. However, disruptions in homeostasis, which may be caused by factors such as host genetics, diet, medications, infections, and circadian rhythm, can contribute to the development of various diseases, including obesity and accelerated aging13. In obese individuals, the microbiome exhibits an increased capacity to extract energy from food compared to individuals with a healthy body weight, potentially leading to higher energy intake, which precedes obesity and the development of metabolic disorders14
Significant changes in the composition of the gut microbiome in obese individuals include alterations in the presence, abundance, and activity of the Bacteroidales order (including species such as Lactobacillus spp., Bifidobacterium spp., Bacteroides spp., and Enterococcus spp.). The ratio of Firmicutes to Bacteroidetes shifts, along with a noted decrease in
Clostridia , including Clostridiumleptum , and Enterobacter spp.15-18. The composition of the gut microbiome also varies depending on the degree of obesity (Table 1). Specifically, significant reductions in bacterial genera such as Akkermansia, Faecalibacterium, Oscillibacter,and Alistipeshave been recorded in obese individuals compared to those with normal body weight14
Higher levels of Lactobacillus reuteri have been associated with obesity, potentially leading to significant weight gain, whereas species such as Bifidobacterium animalis, Methanobrevibacter smithii , and other Lactobacillus species are more abundant in individuals with normal body weight. In contrast, M. smithii levels are lower in obese individuals compared to those with normal weight19. These alterations in gut microbiome composition could serve as early diagnostic markers for treating type 2 diabetes (T2DM) in high-risk patients20. Additional studies have also shown that the gut microbiome can influence glucose metabolism, while certain microbial species, such as Bacteroides faecalis, may accelerate diabetes progression21,22
Diet has a significant impact on the composition of the gut microbiome and represents one of the most important factors influencing changes in bacterial flora24. Research shows that weight loss, induced by a diet with reduced carbohydrate or fat intake, can lead to increased bacterial diversity in the gut and a reduction in chronic systemic inflammation25. Certain dietary patterns are associated with characteristic microbiotic profiles. For instance, an increased presence of the genus Prevotella is linked to a fiber-rich diet, while a diet high in protein is associated with a predominance of the genus Bacteroides25. Additionally, specific dietary components, such as caffeine, omega-3 fatty acids, and green tea can promote the growth of beneficial gut bacteria and positively influence the Firmicutes-to-Bacteroidetes ratio26. Furthermore, the intake of fruits, vegetables, and extra virgin olive oil contributes to positive changes in microbiome composition27
The Mediterranean diet, rich in prebiotics, can also have a beneficial effect on gut microbiome stability24. A clinical study conducted by Zimmer et al. showed that individuals following a vegetarian or vegan diet have significantly lower levels of bacteria from the genera Bacteroides, Bifidobacterium , as well as Escherichia coli and Enterobacteriaceaespecies in their gut microbiota compared to omnivores. Since vegetarian and vegan diets typically contain higher amounts of carbohydrates and dietary fiber, the gut microbiome under these conditions ferments indigestible polysaccharides into short-chain fatty acids (SCFAs), which can have a positive impact on gut health28. Reduced gut microbiome diversity in individuals consuming a Western diet has been associated with an increased prevalence of obesity and related diseases, including coronary vascular disease, metabolic syndrome, and non-alcoholic fatty liver disease (NAFLD)29.
Besides dietary interventions, gut microbiome modulation can also be achieved through probiotics, such as Bifidobacterium and Lactobacillus species,

TABLE 1 Evidence for each ingredient on sperm parameters and/or live birth rates
Upregulated
Overweight pregnant women
Obese women: Bacteroides, Staphylococcus aureus, Enterobacteriaceae, Escherichia coli, Staphylococcus
Excessive weight gain: Escherichia coli, C. leptum, Staphylococcus
Mild weight gain: Clostridium Bifidobacterium
Overweight and obese
Pre-pregnancy and infant
Overweight/obese women in metabolic disorder group
Staphylococcus aureus, Firmicutes-to-Bacteroidetes ratio; Lactobacillus spp. (Lactobacillus reuteri, Lactobacillus), E. coli, Prevotellaceae, Archaea, Firmicutes
Infants whose mothers are overweight: Akkermansia muciniphila, Staphylococcus aureus, Clostridium histolyticum,
Infants of mothers with ex-cessive weight gains: Clostridium histolyticum, Staphylococcus aureus
E. rec-to-Bacto ratio, E. rectale-C coccoides, Gramnegative,Firmicutes/acteroidetesratio
as well as prebiotics like lactulose, inulin, fructooligosaccharides, and galactooligosaccharides, which contribute to balancing the gut ecosystem30,31 Westernization of dietary habits leads to disruptions in gut microbiota composition32. Studies have shown that African children consuming a low-fat, high-fiber diet have greater microbial diversity in their gut and a lower number of pathogenic bacteria. These children also have higher levels of Bacteroidetes compared to European children, who have a higher abundance of Firmicutes and Enterobacteriaceae, such as Shigella and Escherichia33. Conversely, a diet high in fat and low in fiber reduces microbial diversity, decreases the number of protective bacteria, and lowers SCFA production. The consumption of fiber-rich foods, such as fruits, vegetables, and legumes, increases gut microbiota diversity and is also associated with lower weight gain in humans, independent of total energy intake34
One approach to combating obesity and related diseases involves modifying the microbiota of obese individuals to increase the diversity of beneficial microorganisms35. Studies have shown a link between probiotics and weight reduction in both animals and humans36. An inadequate diet in obese individuals can facilitate the extraction of energy from consumed food and promote its storage in the host's adipose tissue. Research suggests that probiotics and synbiotics may contribute to weight reduction through various mechanisms.
Probiotics promote the restoration of tight junctions between epithelial cells, reducing intestinal permeability, preventing bacterial translocation, and alleviating inflammation caused by lipopolysaccharides (LPS). The reduction in inflammation improves insulin sensitivity in the hypothalamus, enhancing
Downregulated
Excessive weight gain: Akkermansia muciniphila, Bifidobacterium
Bacteroidesvulgatus, M. smithii
Bacteroides-Prevotella
the sensation of satiety.
Additionally, increased concentrations of leptin in adipose tissue, glucagon-like peptide 1 (GLP-1), and pancreatic polypeptide (PYY) in the gut contribute to reduced food intake by increasing satiety.
Several bacterial strains have demonstrated positive effects, including reducing endotoxemia, adiposity, tissue inflammation, body weight, leptin (LEP) levels, and overall energy intake. Among the most extensively studied probiotic species are Bifidobacterium and Lactobacillus spp., though their effects on obesity depend on the specific strain and species. The composition of the gut microbiota varies among individuals, but most studies indicate that the Firmicutes-to-Bacteroidetes ratio is significantly higher in obese individuals. Additionally, previous research has shown an increased presence of Bacteroidetes in stool following weight loss, while a higher abundance of Firmicutes has been linked to the development of obesity. Furthermore, studies have demonstrated that overweight and obese individuals with a high Firmicutes/Bacteroides ratio achieve better health outcomes when following a diet rich in fiber and whole grains compared to those with a lower ratio of these bacterial species37
One study examined the use of probiotic species Lactobacillus and Bifidobacterium in obese animals due to their low pathogenicity and high resistance to antibiotic activity38 Bifidobacterium contributed to reducing inflammation, improving insulin sensitivity, and lowering serum cholesterol and triglyceride levels, primarily by decreasing intestinal permeability. Additionally, the application of probiotics containing Lactobacillus in obese animals resulted in effective reductions in body fat mass and improved regulation of blood lipids and glucose, which was attributed to increased fatty acid oxidation and inhibition of lipoprotein lipase activity39. The use of
certain Lactobacillus strains has also been investigated in humans.
A study by Luoto et al. included two groups of children. One group received a placebo, while the other consumed the L. rhamnosus probiotic, which contributed to the regulation of the child's body weight during the first few years of life and during the initial stages of excessive weight gain, though this effect was not observed in later stages of life40,41. Over twelve weeks of application in obese individuals, the Lactobacillus gasseri SBT2055 probiotic contributed to the reduction of abdominal obesity and body weight, while no such effect was seen in the group receiving L. gasseri BNR1742,43. Moreover, in obese children with insulin resistance, the intake of Aspergillus flavus CECT7765 resulted in a significant reduction in body weight44. In adults, Lactobacillus and Bifidobacterium contributed to significant reductions in body weight, BMI, waist circumference, and body fat percentage45.
Oral supplementation with probiotics has proven effective in improving the risk of developing atherosclerosis, reducing LDL cholesterol and total cholesterol levels, as well as optimizing body composition, body weight, and visceral fat42,46. Furthermore, probiotics have antibacterial properties and enhance the gut barrier and immunomodulatory functions, making them beneficial in regulating the gut microbiota and managing obesity and associated diseases45
Prebiotics are food components that are indigestible but can positively affect the host's health by selectively stimulating the growth or activity of specific gut bacteria, thereby improving host health46 Additionally, they can serve as a medium for probiotics, promoting their growth. Examples of prebiotics include inulin, lactulose, fructooligosaccharides, and derivatives of galactose and β-glucans, which could be considered future tools for combating obesity. Oligosaccharides, fructooligosaccharides (FOS), galactooligosaccharides (GOS), and polyphenols are the most widespread prebiotics47
Animal studies have shown that prebiotics, such as fructooligosaccharides and galactooligosaccharides, can alter the gut microbiome composition, increase the number of beneficial bacteria like Bifidobacterium and Lactobacillus , and consequently reduce body weight and fat tissue48,49. Moreover, in humans, prebiotics have also been associated with improvements in gut barrier function and metabolic parameters, including insulin resistance50
Studies investigating prebiotics have shown that these compounds promote positive changes in the composition and function of the gut microbiome. By increasing the number of Bifidobacterium species and other butyrate producers, prebiotics contribute to improved metabolic functions and strengthening the gut barrier against pathogens51,52. Inulin, as a fermentable carbohydrate, can increase the density of cells producing the hormone PYY, which suppresses appetite by 8%, thereby contributing to reduced food intake and potentially playing an important role in the treatment of obesity. Additionally, supplementation with GOS in healthy individuals has been shown

to increase the number of beneficial Bifidobacterium species while simultaneously reducing the presence of Bacteroides53
Furthermore, individual studies indicate that an increase in Bifidobacterium following the addition of FOS can be accompanied by the growth of Lactobacillus species, resulting in a decrease in ghrelin levels, PYY, and overall food intake52,53. The same studies also found that discontinuing prebiotic consumption led to a decrease in Lactobacillus species and changes in certain butyrate-producing microbes such as Faecalibacterium, Ruminococcus, and Oscillospira . The research results showed enhanced microbial diversity and beneficial effects on host health due to prebiotic consumption.
In addition, some studies suggest the prebiotic potential of flavanols from cocoa, dark chocolate (DC), and lycopene, and their impact on the gut microbiome composition. Research conducted on obese subjects revealed that one month of lycopene consumption, either alone or combined with DC, resulted in significant changes in the gut microbiome. A dose-dependent increase in Bifidobacterium species was observed with lycopene supplementation, while Lactobacillus species increased with DC intake. Furthermore, both formulations resulted in a decrease in the Bacteroides genus54
Although these findings suggest potential benefits for the gut microbiome, there is currently insufficient evidence to confirm their significant effect on weight loss.
In addition to diet, physical activity can also influence the composition of the gut microbiome55. Physical activity promotes the growth of beneficial bacteria such as Staphylococcus hominis and Akkermansia muciniphila , which are positively associated with health55
A study by Allen et al. (2018) examined the impact of physical activity on the gut microbiome in lean and obese individuals with a sedentary lifestyle. Participants engaged in a six-week endurance exercise program, which included 30 to 60 minutes of moderate to intense activity. Stool samples were collected
before and after the intervention to analyze changes in the microbiota56
It was observed that physical activity alters the gut microbiome, with differences noted based on obesity status. In lean participants, there was an increase in fecal short-chain fatty acids (SCFAs), such as acetate and butyrate, whereas no such effect was observed in obese participants. Nevertheless, exercise resulted in a reduction in body fat percentage in both groups.
In recent years, therapeutic interventions aimed at modulating the gut microbiome have been developed as a potential approach for treating obesity and related disorders. One such approach is fecal microbiota transplantation (FMT), which is considered an extremely effective method57 .
FMT involves transferring the composition of the gut microbiota from a healthy donor to the patient’s gastrointestinal tract, typically through duodenal endoscopy or colonoscopy, with the goal of normalizing the structure and function of the gut microbial community. The compatibility between the donor and the recipient must be considered, as it is crucial for the successful establishment of the donor's microbial strains in the recipient’s gut. FMT has shown over 90% effectiveness in treating Clostridiumdifficile infections57
Research shows that while healthy adults have a relatively stable and balanced gut microbiome, individuals with diet-induced obesity exhibit significant variations in their microbiome composition compared to individuals with normal body weight.
Changes in the gut microbiome of obese individuals, including an increased ability to extract energy from food and a reduction in bacterial species diversity, may contribute to the development of metabolic disorders. On the other hand, dietary interventions, especially those focusing on increased fiber, prebiotics, and probiotics intake, have proven effective tools for modulating the microbiome and improving metabolic health. In addition to diet, physical activity positively influences the microbiome by promoting the growth of beneficial bacteria associated with health. New therapeutic approaches, such as fecal microbiota transplantation (FMT), also open possibilities for innovative treatments for obesity.
In conclusion, physical activity induces structural and functional changes in the human gut microbiome, with these changes conditioned by obesity status and continuous exercise, regardless of dietary habits. However, it is still uncertain whether exercise confers health benefits by modifying the gut microbiome, as well-designed controlled trials are lacking. Given its observed satisfactory effects, FMT is expected to have potential as a therapy for restoring and improving gut microbiome functionality, which could help treat various diseases related to gut dysbiosis. These include chronic constipation, irritable bowel syndrome, Crohn’s disease, and ulcerative colitis. Additionally, increasing research suggests that FMT may also have potential in treating obesity and related disorders, including type 2 diabetes. Due to its numerous effects on human health, Akkermansia muciniphila is considered a promising prebiotic for improving obesity outcomes.
Ultimately, the approach to obesity must be multidisciplinary, involving changes in lifestyle, diet, and targeted interventions on the gut microbiome to achieve long-term sustainable results in weight management and reduction of metabolic disease risk. Focusing on the composition of the gut microbiome may represent a promising treatment for obesity. However, further research is needed to confirm the effects and effectiveness of this therapeutic approach.
1 Asadi A, Shadab Mehr N, Mohamadi MH, Shokri F, Heidary M, Sadeghifard N, Khoshnood S. Obesity and gut-microbiota-brain axis: A narrative review. J Clin Lab Anal. 2022 May;36(5):e24420. doi: 10.1002/jcla.24420. Epub 2022 Apr 14. PMID: 35421277; PMCID: PMC9102524.
2 WHO – World Health Organization (2021b) WHO Guideline on the integrated management of children and adolescents with obesity: a primary health care approach, World Health Organization, https://www.who.int/news-room/articles-detail/ call-for-experts-who-guideline-development-group-treatmentof-children-and-adolescents-with-obesity. Pristupljeno 20. veljače 2025.
3 V Calcaterra, H Cena, V Rossi, S Santero, A Bianchi, G Zuccotti (2023) Ultra-Processed Food, Reward System and Childhood Obesity. Children (Basel) 10, 804. doi: 10.3390/children10050804.
4 V Rahelić (2021) Uloga nutritivne intervencije u multidisciplinarnom pristupu liječenja pretilosti u djece i adolescenata. Doktorska disertacija. Zagreb: Sveučilište u Zagrebu, Prehrambeno-biotehnološki fakultet.
5 WHO-World Health Organization (2021a) Obesity and overweight, World Health Organization, https://www.who.int/news-room/ fact-sheets/detail/obesity-and-overweight. Pristupljeno 20. veljače 2025.
6 L. Crovesy, M. Ostrowski, D.M.T.P. Ferreira, E.L. Rosado, M. Soares-Mota (2017). Effect of Lactobacillus on body weight and body fat in overweight subjects: a systematic review of randomized controlled clinical trials. Int. J. Obes., 41 (11), 1607-1614.
7 B.A. Peters, J.A. Shapiro, T.R. Church, G. Miller, C. Trinh-Shevrin, E. Yuen, C. Friedlander, R.B. Hayes, J. Ahn (2018). A taxonomic signature of obesity in a large study of American adults. Sci. Rep., 8 (1), 9749.
8 C.L. Boulangé, A.L. Neves, J. Chilloux, J.K. Nicholson, M.E. Dumas (2016). Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med., 8 (1), 42.
9 Asadi A, Shadab Mehr N, Mohamadi MH, Shokri F, Heidary M, Sadeghifard N, Khoshnood S. Obesity and gut-microbiota-brain axis: A narrative review. J Clin Lab Anal. 2022 May;36(5):e24420. doi: 10.1002/jcla.24420.
10 J. Liang, M. Zhang, X. Wang, Y. Ren, T. Yue, Z. Wang, Z. Gao (2021). Edible fungal polysaccharides, the gut microbiota, and host health. Carbohydr. Polym., 273, Article 118558.
11 H. Tilg, N. Zmora, T.E. Adolph, E. Elinav (2020). The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol., 20 (1), 40-54.
12 H.Y. Cheng, M.X. Ning, D.K. Chen, W.T. Ma (2019). Interactions between the gut microbiota and the host innate immune response against pathogens. Front. Immunol., 10, 607.
13 S. Lynch, O. Pedersen (2016). The human intestinal microbiome in health and disease N. Engl. J. Med., 375 (24), 2369-2379
14 L.B. Thingholm, M.C. Rühlemann, M. Koch, B. Fuqua, G. Laucke, R. Boehm, C. Bang, E.A. Franzosa, M. Hübenthal, A. Rahnavard, F. Frost, J. Lloyd-Price, M. Schirmer, A.J. Lusis, C.D. Vulpe, M.M. Lerch, G. Homuth, T. Kacprowski, C.O. Schmidt, U. Nöthlings, T.H. Karlsen, W. Lieb, M. Laudes, A. Franke, C. Huttenhower (2019). Obese individuals with and without type 2 diabetes show different gut microbial functional capacity and composition. Cell Host Microbe, 26 (2), 252-264.
15 C.B. de La Serre, C.L. Ellis, J. Lee, A.L. Hartman, J.C. Rutledge, H.E. Raybould
16 D. Chen, Z. Yang, X. Chen, Y. Huang, B. Yin, F. Guo, H. Zhao, J. Huang, Y. Wu, R. Gu (2015). Effect of Lactobacillus rhamnosus hsryfm 1301 on the gut microbiota and lipid metabolism in rats fed a high-fat diet. J. Microbiol. Biotechnol., 25 (5), 687-695.
17 K.A. Kim, W. Gu, I.A. Lee, E.H. Joh, D.H. Kim (2012). High fat diet-induced gut microbiota exacerbates inflammation and
obesity in mice via the TLR4 signaling pathway PLoS One, 7 (10), Article e47713.
18 M.K. Hamilton, G. Boudry, D.G. Lemay, H.E. Raybould (2015). Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am. J. Physiol. Gastrointest. Liver Physiol., 308 (10), G840-G851.
19 M. Million, M. Maraninchi, M. Henry, F. Armougom, H. Richet, P. Carrieri, R. Valero, D. Raccah, B. Vialettes, D. Raoult (2012). Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes., 36 (6), 817-825.
20 B. Wei, Y. Wang, S. Xiang, Y. Jiang, R. Chen, N. Hu (2021). Alterations of gut microbiome in patients with type 2 diabetes mellitus who had undergone cholecystectomy. Am. J. Physiol. Endocrinol. Metab., 320 (1), E113-E121.
21 K.M. Utzschneider, et al. (2016). Mechanisms linking the gut microbiome and glucose metabolism (vol. 101, pg 1445, 2016). J. Clin. Endocrinol. Metab., 101 (6), 2622-2622.
22 L. Brunkwall, M. Orho-Melander (2017). The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia, 60 (6), 943-951.
23 G Jiafeng, N Qingqiang, S Wei, L Liangge F Xiujing (2022). The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother., 147, 112678.
24 A. Beam, E. Clinger, L. Hao (2021). Effect of diet and dietary components on the composition of the gut microbiota. Nutrients, 13 (8), 2795.
25 K.E. Bouter, D.H. van Raalte, A.K. Groen, M. Nieuwdorp (2017). Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction Gastroenterology, 152 (7), 1671-1678.
26 L.H.S. Lau, S.H. Wong (2018). Microbiota, obesity and NAFLD. Adv. Exp. Med. Biol., 1061, 111-125.
27 A. Pascale, N. Marchesi, C. Marelli, A. Coppola, L. Luzi, S. Govoni, A. Giustina, C. Gazzaruso (2018). Microbiota and metabolic diseases. Endocrine, 61 (3), 357-371.
28 J. Zimmer, B. Lange, J.S. Frick, H. Sauer, K. Zimmermann, A. Schwiertz, K. Rusch, S. Klosterhalfen, P. Enck (2012). A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr., 66 (1), 53-60.
29 Y. Fan, O. Pedersen (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol., 19 (1), 55-71.
30 L. Crovesy, M. Ostrowski, D.M.T.P. Ferreira, E.L. Rosado, M. Soares-Mota (2017). Effect of Lactobacillus on body weight and body fat in overweight subjects: a systematic review of randomized controlled clinical trials. Int. J. Obes., 41 (11), 1607-1614.
31 X. Gao, Y. Zhu, Y. Wen, G. Liu, C. Wan (2016). Efficacy of probiotics in non-alcoholic fatty liver disease in adult and children: a meta-analysis of randomized controlled trials. Hepatol. Res., 46 (12), 1226-1233.
32 Aoun A, Darwish F, Hamod N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev Nutr Food Sci. 2020 Jun 30;25(2):113-123. doi: 10.3746/pnf.2020.25.2.113
33 De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14691-6. doi: 10.1073/pnas.1005963107
34 Aoun A, Darwish F, Hamod N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev Nutr Food Sci. 2020 Jun 30;25(2):113-123. doi: 10.3746/pnf.2020.25.2.11
35 H.Y. Li, D.D. Zhou, R.Y. Gan, S.Y. Huang, C.N. Zhao, A. Shang, X.Y. Xu, H.B. Li (2021). Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: a narrative revie. Nutrients, 13 (9) (2021), 3211.
36 Asadi A, Shadab Mehr N, Mohamadi MH, Shokri F, Heidary M, Sadeghifard N, Khoshnood S. Obesity and gut-microbiota-brain axis: A narrative review. J Clin Lab Anal. 2022 May;36(5):e24420. doi: 10.1002/jcla.24420.
37 Aoun A, Darwish F, Hamod N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev Nutr Food Sci. 2020 Jun 30;25(2):113-123. doi: 10.3746/pnf.2020.25.2.113.
38 T. Cerdó, J.A. García-Santos, M. G Bermúdez, C. Campoy (2019). The role of probiotics and prebiotics in the prevention and treatment of obesity. Nutrients, 11 (3).
39 P. Gerard (2016). Gut microbiota and obesity. Cell. Mol. Life Sci., 73 (1), 147-162.
40R. Luoto, M. Kalliomäki, K. Laitinen, E. Isolauri (2010). The impact of perinatal probiotic intervention on the development of overweight and obesity: follow-up study from birth to 10 years. Int. J. Obes., 34 (10), 1531-1537.
41 G Jiafeng, N Qingqiang, S Wei, L Liangge F Xiujing (2022). The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother., 147, 112678.
42 Y. Kadooka, M. Sato, K. Imaizumi, A. Ogawa, K. Ikuyama, Y. Akai, M. Okano, M. Kagoshima, T. Tsuchida (2010). Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr., 64 (6) 636-643.
43 S.P. Jung, K.M. Lee, J.H. Kang, S.I. Yun, H.O. Park, Y. Moon, J.Y. Kim (2013). Effect of Lactobacillus gasseri BNR17 on overweight and obese adults: a randomized, double-blind clinical trial. Korean J. Fam. Med., 34 (2), 80-89.
44 J. Sanchis-Chordà, E. Del Pulgar, J. Carrasco-Luna, A. Benítez-Páez, Y. Sanz, P. Codoñer-Franch (2019). Bifidobacterium pseudocatenulatum CECT 7765 supplementation improves inflammatory status in insulin-resistant obese children. Eur. J. Nutr., 58 (7), 2789-2800.
45 L. Abenavoli, E. Scarpellini, C. Colica, L. Boccuto, B. Salehi, J. Sharifi-Rad, V. Aiello, B. Romano, A. De Lorenzo, A.A. Izzo, R. Capasso (2019). Gut microbiota and obesity: a role for probiotics. Nutrients, 11 (11).
46 H.S. Ejtahed, J. Mohtadi-Nia, A. Homayouni-Rad, M. Niafar, M. Asghari-Jafarabadi, V. Mofid (2012). Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition, 28 (5), 539-543.
47 P. Gerard (2016). Gut microbiota and obesity. Cell. Mol. Life Sci., 73 (1), 147-162.
48 Asadi A, Shadab Mehr N, Mohamadi MH, Shokri F, Heidary M, Sadeghifard N, Khoshnood S. Obesity and gut-microbiota-brain axis: A narrative review. J Clin Lab Anal. 2022 May;36(5):e24420. doi: 10.1002/jcla.24420
49 A. Adak, M.R. Khan (2019). An insight into gut microbiota and its functionalities. Cell Mol. Life Sci., 76 (3), 473-493.
50 A. Everard, V. Lazarevic, M. Derrien, M. Girard, G.G. Muccioli, A.M. Neyrinck, S. Possemiers, A. Van Holle, P. François, W.M. de Vos, N.M. Delzenne, J. Schrenzel, P.D. Cani (2011). Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes, 60 (11), 2775-2786.
51 J.A. Parnell, R.A. Reimer (2009). Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr., 89 (6), 1751-1759.
52 Yoo S, Jung SC, Kwak K, Kim JS. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int J Mol Sci. 2024 Apr 29;25(9):4834. doi: 10.3390/ijms25094834
53 Krumbeck JA, Rasmussen HE, Hutkins RW, Clarke J, Shawron K, Keshavarzian A, Walter J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome. 2018 Jun 28;6(1):121. doi: 10.1186/ s40168-018-0494-4
54 Wiese M, Bashmakov Y, Chalyk N, Nielsen DS, Krych Ł, Kot W, Klochkov V, Pristensky D, Bandaletova T, Chernyshova M, Kyle N, Petyaev I. Prebiotic Effect of Lycopene and Dark Chocolate on Gut Microbiome with Systemic Changes in Liver Metabolism, Skeletal Muscles and Skin in Moderately Obese Persons. Biomed Res Int. 2019 Jun 2;2019:4625279. doi: 10.1155/2019/4625279.
55 C. Bressa, M. Bailén-Andrino, J. Pérez-Santiago, R. González-Soltero, M. Pérez, M.G. Montalvo-Lominchar, J.L. Maté-Muñoz, R. Domínguez, D. Moreno, M. Larrosa (2017). Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One, 12 (2), Article e0171352.
56 Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD, Woods JA. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc. 2018 Apr;50(4):747-757. doi: 10.1249/MSS.0000000000001495.
57 P. de Groot, T. Scheithauer, G.J. Bakker, A. Prodan, E. Levin, M.T. Khan, H. Herrema, M. Ackermans, M.J.M. Serlie, M. de Brauw, J.H.M. Levels, A. Sales, V.E. Gerdes, M. Ståhlman, A.W.M. Schimmel, G. Dallinga-Thie, J.J. Bergman, F. Holleman, J.B.L. Hoekstra, A. Groen, F. Bäckhed, M. Nieuwdorp (2020). Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut, 69 (3), 502-512

Butyric acid and sodium butyrate have emerged as promising approaches in mitigating gut inflammatory diseases by restoring gut microbiome balance and modulating immune responses. The challenge of effective butyrate delivery remains a significant barrier, as its poor stability and rapid absorption limit its ability to reach the colon, where it exerts its most beneficial effects.
AUTHOR: Valerija Pandža, MPharm
The gut microbiome and its metabolic byproducts play a crucial role in maintaining host health and homeostasis. One such critical metabolite is butyrate, a short-chain fatty acid produced through the fermentation of dietary fiber by specific gut bacterial taxa. Butyrate is known to exert a wide range of beneficial effects on the host, including serving as a primary energy source for intestinal epithelial cells, modulating the immune system, and influencing diverse metabolic pathways throughout the body.
Recent research has highlighted the potential of butyrate and its derivatives, such as butyric acid and sodium butyrate, in mitigating gut inflammatory diseases. Inflammatory bowel diseases, including Crohn's disease and ulcerative colitis, are chronic, debilitat-
ing conditions characterized by persistent inflammation of the gastrointestinal tract. Depletion of butyrate-producing taxa in the gut microbiome has been linked to the development of these conditions, as well as other noncommunicable diseases like type 2 diabetes, obesity, and cardiovascular disease. The biological significance of butyrate metabolism by the intestinal microbiome is multifaceted. Butyrate can cross the intestinal epithelium and reach the lamina propria and peripheral blood, where it can exert potent immunomodulatory effects. Specifically, butyrate has been shown to limit the effector function of natural killer cells, a key component of the innate immune system
A crucial event in the early stages of chronic gut inflammatory diseases is the dysregulated recruit-
ment and excessive accumulation of immune cells in the gut laminapropria . The excessive secretion of pro-inflammatory factors and persistent hyperactive inflammatory responses follow this. Increasing evidence suggests that butyric acid (BA) or sodium butyrate (NaB) may help alleviate gut inflammatory diseases.
There is also a challenge of effective delivery for butyrate supplementation: While it has shown positive effects on gut health, it may not always reach the site where it is needed.
Butyrate, a short-chain fatty acid (SCFA), is an essential metabolite generated in the colon by fermenting dietary fibers through gut microbiota. It particularly plays an important role in maintaining intestinal health, supporting epithelial barrier functions, modulating immune responses and regulating inflammatory processes. For these reasons, butyrate supplementation has been a subject of interest, especially so for those with sub-optimal gut microbiota or inflammatory bowel diseases. However, one important aspect of butyrate supplementation is a proper delivery to the gut as butyrate has poor stability, absorption, and bioavailability.
Butyrate serves multiple critical functions in the gut. It is the primary energy source for colonocytes, promoting epithelial cell integrity and reducing the risk of leaky gut syndrome. Additionally, it exerts an
ti-inflammatory effects by modulating the immune system, inhibiting the activation of nuclear factorkappa B (NF-κB), and reducing the production of pro-inflammatory cytokines. Butyrate also enhances the expression of tight junction proteins, reinforcing the intestinal barrier and preventing the translocation of harmful pathogens. Furthermore, it supports beneficial gut bacteria, creating an environment less favorable for pathogenic microbes, and acts as a histone deacetylase (HDAC) inhibitor, influencing gene expression related to inflammation, apoptosis, and metabolism.
Despite these benefits, ensuring that butyrate reaches the gut effectively without being prematurely degraded or absorbed in the upper gastrointestinal (GI) tract remains to be challenging.
One of the primary challenges is the chemical instability and rapid absorption of butyrate. It is highly volatile and prone to oxidation, which affects its stability in conventional supplement forms. Free butyrate is rapidly absorbed in the stomach and upper small intestine, making it difficult to reach the colon, where it is most needed. This limitation significantly reduces its effectiveness as an oral supplement. Another issue is the unpleasant odor and taste of pure

barrier to consumer acceptance.
To overcome premature absorption, various encapsulation techniques have been explored. Microencapsulation involves coating butyrate with lipid or polymer-based materials to protect it from early absorption and ensure targeted release in the colon. Enteric coatings, which are pH-sensitive, prevent dissolution in the stomach but allow release in the intestines. However, variability in individual gut pH can influence efficacy. Another approach is the use of prodrugs and conjugated forms, where butyrate is esterified with glycerol or amino acids to enhance stability and delay absorption. However, metabolism rates vary among individuals, affecting the overall effectiveness of these strategies.
Establishing an optimal dosage for butyrate supplementation is another challenge. Too little may be ineffective, while excessive intake could lead to gastrointestinal discomfort. Furthermore, bioavailability varies based on formulation, dietary habits, and individual microbiota composition. The presence of specific bacterial strains is necessary for efficient butyrate production and utilization. Some individuals with dysbiosis (imbalanced gut microbiota) may not respond well to butyrate supplementation due to a lack of appropriate bacterial populations to metabolize it effectively.
Advances in butyrate delivery technologies continue to improve efficacy. Lipid-based formulations provide sustained release and improved gut targeting. Additionally, precision medicine approaches, including personalized gut microbiome analysis, could help tailor butyrate supplementation strategies based on an individual's microbial composition.
While butyrate supplementation holds great promise for gut health, its effective delivery remains
challenging. Overcoming issues related to stability, bioavailability, and targeted release is crucial for maximizing its therapeutic benefits. Addressing these challenges ensures that butyrate reaches its intended site of action in the gut, enhancing its potential as a therapeutic agent for gastrointestinal health.
Chriett, S., Dąbek, A., Wojtala, M., Vidal, H., Balcerczyk, A., & Pirola, L. (2019). Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. In Scientific Reports (Vol. 9, Issue 1). Nature Portfolio. https://doi.org/10.1038/s41598-018-36941-9
Kasarełło, K., Cudnoch-Jędrzejewska, A., & Czarzasta, K. (2023). Communication of gut microbiota and brain via immune and neuroendocrine signaling [Review of Communication of gut microbiota and brain via immune and neuroendocrine signaling]. Frontiers in Microbiology, 14. Frontiers Media. https://doi.org/10.3389/fmicb.2023.1118529
Puertollano, E., Kolida, S., & Yaqoob, P. (2014). Biological significance of short-chain fatty acid metabolism by the intestinal microbiome [Review of Biological significance of short-chain fatty acid metabolism by the intestinal microbiome]. Current Opinion in Clinical Nutrition & Metabolic Care, 17(2), 139. Lippincott Williams & Wilkins. https://doi.org/10.1097/mco.0000000000000025
Vital, M., Karch, A., & Pieper, D. H. (2017). Colonic Butyrate-Producing Communities in Humans: an Overview Using Omics Data. In mSystems (Vol. 2, Issue 6). American Society for Microbiology. https://doi.org/10.1128/msystems.00130-17
Zaiatz-Bittencourt, V., Jones, F., Tosetto, M., Scaife, C., Cagney, G., Jones, E., Doherty, G. A., & Ryan, E. J. (2023). Butyrate limits human natural killer cell effector function. In Scientific Reports (Vol. 13, Issue 1). Nature Portfolio. https://doi.org/10.1038/s41598-023-29731-5
Facchin, S., Vitulo, N., Calgaro, M., Buda, A., Romualdi, C., Pohl, D., Perini, B., Lorenzon, G., Marinelli, C., D’Incà, R. and Sturniolo, G.C., 2020. Microbiota changes induced by microencapsulated sodium butyrate in patients with inflammatory bowel disease. Neurogastroenterology & Motility, 32(10), p.e13914.
Peng, L., Li, Z. R., Green, R. S., Holzman, I. R., & Lin, J. (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. The Journal of nutrition, 139(9), 1619–1625. https://doi.org/10.3945/jn.109.104638













sabinsa.eu
info@sabinsa.eu
+49 6103 270 1111







BioPerine®, a standardized extract of Piper nigrum containing 95% piperine, stimulates thermogenic action in the epithelial cells of the small intestine. This acts as a thermonutrient that allows for increased absorption and bioavailability of nutrients. BioPerine is the only product made from black pepper to obtain the original patented status for its ability to increase the bioavailability of nutritional compounds. It is the only branded black pepper extract to have undergone extensive clinical studies to substantiate its safety and efficacy for nutritional use.
These statements


BioPerine®, a standardized extract of Piper containing 95% piperine, stimulates thermogenic the epithelial cells of the small intestine. This acts thermonutrient that allows for increased absorption bioavailability of nutrients. BioPerine is the only made from black pepper to obtain the original patented status for its ability to increase the bioavailability nutritional compounds. It is the only branded black extract to have undergone extensive clinical studies substantiate its safety and efficacy for nutritional use.
BioPerine®, a standardized extract containing 95% piperine, stimulates the epithelial cells of the small intestine. thermonutrient that allows for increased bioavailability of nutrients. BioPerine made from black pepper to obtain status for its ability to increase nutritional compounds. It is the only extract to have undergone extensive substantiate its safety and efficacy for evaluated by the
This product is not intended to diagnose, treat, cure, mitigate or prevent any disease.
As the demand for high-quality, effective supplements continues to rise, companies that embrace innovation will lead the way. MicroActive® nutrients represent a game-changing opportunity for developers committed to excellence. With pioneering companies like Valeovita setting the standard, the future of food supplements looks brighter than ever.

The founder of Valeovita, Mrs. Mirjana Franković
In the fast-paced world of dietary supplements, innovation and efficiency are crucial, and as the market becomes increasingly saturated, the need to adopt cutting-edge technologies becomes even more important. One such discovery is MicroActive® – a range of patented nutrient delivery systems. MicroActive® advanced formulations ensure gradual release, enhanced bioavailability, and consistent performance, making them the top choice in supplement development. MicroActive® Nutrients was developed by Bioactives USA and is distributed by the Slovenian company Valeovita Ltd.
MicroActive® nutrients are engineered to address some of the most pressing challenges in supplement formulation: poor bioavailability and inconsistent absorption rates. Many traditional supplements fail to deliver their active ingredients effectively, resulting in wasted potential and reduced benefits for consumers. By contrast, MicroActive® utilizes proprietary micronization and encapsulation technologies to ensure:
• Sustained release: Active ingredients are relea-
sed gradually over time, maintaining therapeutic levels in the body for longer periods.
• Improved absorption: The unique structure enhances the solubility and bioavailability of nutrients, ensuring optimal delivery.
• Versatile applications: MicroActive® formulations can be used in a wide range of products, including capsules, powders, and functional foods.
These innovations make MicroActive® ideal for addressing the diverse needs of today’s health-conscious consumers.
The patented MicroActive® process (U.S. 7,030,102) complexes each CoQ10 molecule with β-cyclodextrin molecule in a water media. β-cyclodextrin is used extensively in the food and pharmaceutical industries. It is a GRAS compound formed through an enzymatic conversion of starch. One end of the molecule is fat-soluble holding the CoQ10 and the other side is water-soluble making the efficient transport through digestive systems.
When the nano-sized MicroActive® complex ar-



rives at the cell, it comes apart depositing a single CoQ10 molecule for efficient absorption.
The value proposition for food supplement developers
Incorporating MicroActive® nutrients into your formulations offers numerous advantages:
1. Enhanced consumer trust: Products that deliver on their promises foster loyalty and positive wordof-mouth.
2. Regulatory compliance: MicroActive® ingredients meet stringent quality and safety standards, simplifying the approval process across global markets.
3. Cost-effectiveness: Improved bioavailability means lower dosages are required to achieve desired effects, reducing material costs.
4. Market differentiation: Leveraging patented technology sets your brand apart in a competitive landscape.
Valeovita Ltd.: a commitment to excellence
Valeovita Ltd., headquartered in Slovenia, is a trailblazer in the field of hydration and nutrition. Founded by Mirjana Franković, the company has gained a stellar reputation for its dedication to developing high-quality ingredients and solutions that cater to modern wellness demands. By partnering with leading innovators like Bioactives USA, Valeovita ensures that its product offerings consistently exceed industry standards.
Mirjana Franković’s vision is rooted in a deep understanding of the science behind nutrition and the importance of delivering tangible results to consumers. This commitment has positioned Valeovita as a trusted name in the European market and beyond, with products that emphasize efficacy, safety, and sustainability.
The success of Valeovita’s products demonstrates the transformative potential of MicroActive® nutrients. By integrating these advanced delivery systems, the company has developed solutions that resonate with consumers looking for reliable, science-backed health supplements. Whether it’s enhancing hydration, boosting energy, or supporting overall wellness, the combination of MicroActive® technology and Valeovita’s expertise creates undeniable value.
As the demand for high-quality, effective supplements continues to rise, companies that embrace innovation will lead the way. MicroActive® nutrients represent a game-changing opportunity for developers committed to excellence. With pioneering companies like Valeovita d.o.o. setting the standard, the future of food supplements looks brighter than ever.
To learn more about MicroActive® technology as well as for insights into Valeovita’s innovative approach, explore their offerings visit Valeovita. Together, these industry leaders are shaping the future of nutrition-one breakthrough at a time.

Valeovita Ltd. Parmova ulica 53, 1000 Ljubljana, Slovenia www.valeovita.si
T. +386 40 520 257, +385 91 6212 468
E-mail: mirjana.frankovic@valeovita.si office@valeovita.si
This extract brings a deep dive into omega-3 supplement users. 2025 results will be available soon.
AUTHOR: Len Monheit, CEO, Industry Transparency Center
Industry Transparency Center (ITC) is a data, strategy and insights company focused on the health ingredients and natural product sectors globally. With a deep background in strategy, sourcing and supply chain the team at ITC works with industry, consumers and influencers to understand values,
behaviors, purchase drivers to understand and predict trends and guide its education programs for maximum impact. The team produces virtual conferences highlighting emerging and validated nutrition science, driving its application in industry settings.

Key Insight:
For the most part, the response rate for a willingness to take a supplement track with reporting of a health concern.
Consistently though, there seems to be more of a willingness to consider taking supplements for a reported health concern than actually taking supplements for said concern.
Question:
“Would you consider supplements for any of the fol-
lowing health concerns?” Results shown for top 10 health concerns.
Question: “Which of the following health conditions or concerns currently impact or impacted you within the past year?”
Note: US n=733, UK n=407, DE n=385, IT n=404, KR n=448, AU n=338, IN n=468. Entire bar represents total reporting, solid bar represents a willingness to use supplements for that concern, smaller hatch indicates willingness to fulfill.
For the most part, the response rate for a willingness to take a supplement track with reporting of a health concern.
Consistently though, there seems to be more of a willingness to consider taking supplements for a reported health concern than actually taking supplements for said concern.
Question:
“Would you consider supplements for any of the fol-
lowing health concerns?” Results shown for top 10 health concerns. Question: “Which of the following health conditions or concerns currently impact or impacted you within the past year?
Note: US n=733, UK n=407, DE n=385, IT n=404, KR n=448, AU n=338, IN n=468. Entire bar represents total reporting, solid bar represents a willingness to use supplements for that concern, smaller hatch indicates willingness to fulfill.
Key Insight: Omega-3 familiarity is high for all supplement users, sitting at 24% top familiarity and 54% top-2 (sixth among all supplements surveyed).
Question: “How familiar would you consider yourself regarding the use of these supplements?”
Note: All Respondents n=4198
Note: All Respondents n=4198. Question: “How familiar would you consider yourself regarding the use of these supplements?”
Key Insight: Italian and Indian respondents noticeably over-index for a recommendation from a health care professional.
We also see sharp over-indexing from South Korean respondents for proof of safety.
Question: “What are the most important attributes you look for when purchasing supplements?”
Note: US n=733, UK n=407, DE n=385, IT n=404, KR n=448, AU n=338, IN n=468, All Respondents n=4198
Key Insight: Most responses are in 'sometimes' and 'frequently' influences, but Indian respondents over-index for always at 39%, the US is second there at 20%.
Question:

“When deciding which supplements to purchase, to what degree does the sustainability/environmental
impact of a supplement ingredient influence your purchasing decision?”
Note: US n=733, UK n=407, DE n=385, IT n=404, KR n=448, AU n=338. IN n=468, All Respondents n=4198.
Key Insight: • Most responses are in 'sometimes' and 'frequently' influences, but Indian respondents over-index for always at 39%, the US is second there at 20%.
Always influences Frequently influences Sometimes influences Never influences my decision I wasn't aware this was an issue with supplements
Note: US n=733, UK n=407, DE n=385, IT n=404, KR n=448, AU n=338. IN n=468, All Respondents n=4198. Question: “When deciding which supplements to purchase, to what degree does the sustainability/environmental impact of a supplement ingredient influence your purchasing decision?”
Key Insight: Each positive influence response sees high engagement from every group with top responses in either frequently or sometimes. Females over 55 over-index for not being aware of an issue.
Question: “When deciding which supplements to purchase, to what degree does the sustainability/environmental impact of a supplement ingredient influence your purchasing decision?” (Figure 6.)
Note: Female 18-34 n=498, Male 18-34 n=480, Female 35-54 n=634, Male 35-54 n=719, Female 55+ n=373, Male 55+ n=482.
Key Insight:
Responses are relatively similar with a few sharp outliers such as the US for heart health, South Koreans for immune health, and Indian users for Immune health and hair, skin and nails. UK and Australian respondents over-index for joint health.
Question: „Why they take omega-3 supplements?“
Note: US n=303, UK n=247, DE n=209, IT n=205, KR n=156, AU n=202. IN n=65
US UK DE IT KR AU IN
Key Insight:
Consumers are overwhelmingly simply looking for supplements labeled as omega-3, the top response by a decent margin from each respondent group. The second highest response in South Korea, by a decent margin is contains at least 500mg of EPA and DHA.
Question: “When you select what omega-3 product to purchase, do you look for any of these specific characteristics?”
Note: US n=280, UK n=236, DE n=197, IT n=191, KR n=140, AU n=181. IN n=60.
Key Insights:
Either gelatin soft gel or gelatin hard capsule is the top response from each group. As with other categories, the gummy format is driven by the US.
Question: “How do you prefer to consume your omega-3s?”
Note: US n=280, UK n=236, DE n=197, IT n=191, KR n=140, AU n=181. IN n=60.
Key Insight:
Either a recommendation from a health care professional or from a friend/family member are the top responses from each group. Korean respondents significantly over-index for health tv shows.
Question: “How did you learn about omega-3s?”
Note: US n=280, UK n=236, DE n=197, IT n=191, KR n=140, AU n=181





The omega-3 industry continues to evolve, shaped by consumer demand, scientific advancements, regulatory shifts, and supply chain challenges. To provide a comprehensive look at the state of the market, NMC Magazine presents the Omega Industry Expert Panel, featuring insights from leading voices in the sector.

From market growth and emerging trends to supply chain disruptions and regulatory updates, our panelists - Ellen Schutt (GOED), Ståle Søfting (GC Rieber VivoMega), Liki von Oppen-Bezalel (TriNutra), and Tanja Kokkinis (Pharmako Biotechnologies) - offer their perspectives on the key factors shaping the omega-3 landscape in 2025 and beyond.
Scientific research continues to affirm the benefits of omega-3s in heart, brain, eye, and maternal health, though experts emphasize the need for more large-scale and primary prevention studies. On the regulatory front, Europe’s evolving stance on mineral oil aromatic hydrocarbons (MOAH) and the potential impact of U.S. trade policies are key considerations for industry stakeholders.
As sustainability, innovation, and scientific validation drive the industry forward, our expert panel explores the challenges and opportunities that lie ahead for omega-3 producers, suppliers, and consumers worldwide.

“Overall, the EPA and DHA omega-3 supplement market has been strong globally, with growth of 9% in 2023, to reach US$5.6 billion. Mature markets like Europe and the US (as well as Japan and Australia) increased slightly, while China and Southeast Asia and emerging markets in the Rest of the World and Latin America posted higher growth figures.
“In terms of sources, while anchovy oil continues to be the largest source of EPA and DHA oil for the supplement industry, a disruption in the fishing seasons in Peru in 2023 led to a shortage in supply, and concerns about the long-term supply situation. This led to increased interest in alternative supply sources, which continues today. One of the beneficiaries of this situation has been microalgal oils; however, this is still a very small part of the market, and market growth is hindered by the limited availability of both EPA and DHA in algal form. On the topic of delivery forms, the category is expanding beyond traditional soft gel products, with heightened interest in forms like gummies and chews.
The science supporting the benefits of EPA and DHA is strong, with 50,000 published papers and 5000 human clinicals. The strongest science is in heart health, brain health, eye health and prenatal/ maternal health outcomes. The challenge has been the recent lack of large-scale studies and an ongoing need for more primary prevention studies.
From a regulatory perspective, the primary conversation in Europe recently has been an impending European regulation on mineral oil aromatic hydrocarbons (MOAH), a lubricant sometimes used in the manufacture of soft gels, and a potential carcinogen. The EU initially proposed a maximum limit of 2 mg/kg but last year announced a new draft proposal that raises the limit to 10 mg/kg, set to go into effect in 2027. The new limit still requires a vote by European Member States and the European Parliament, which is expected to happen sometime this year.”

“Omega-3s are among the most popular and clinically backed dietary supplements, and the category is expected to continue to gain traction due to innovations such as omega-3 alternatives and synergistic ingredient combinations. These ingredient synergies offer great potential for supporting wellness and lifestyle goals. They can improve efficacy by mutually enhancing the effects of the ingredients and giving consumers more flexibility to customize and incorporate the nutritional products they want to consume.
At TriNutra, we've invested in clinical testing to understand how our premium, USP-grade black seed oil, ThymoQuin, interacts with omega-3s DHA & EPA to enhance their benefits. Our research found that ThymoQuin nearly tripled omega-3s anti-inflammatory activities. Moreover, another published study showed that combining ThymoQuin and Omega-3s favorably affected whole-body energy metabolism. Notably, it significantly balanced blood glucose, insulin sensitivity, and lipid metabolism and lowered cortisol levels significantly more than omega-3 alone, offering comprehensive, synergistic health benefits.”
Ståle Søfting, Sales and Marketing Director
“General consumer interest in omega-3 ingredients remains strong into 2025 despite the turmoil and challenges in fish oil supply and cost over the past couple of years. We expect to see continued changes at the consumer level as well as the production side of omega-3 as new raw materials, such as algae, are developing their position as an alternative source rich in omega-3.
GC Rieber VivoMega will continue expanding our portfolio of EPA and DHA-rich algae ingredients for supplement formulation to complement our strong position in fish oil concentrates, all delivered under the VivoMega quality platform that our customers are used to.”

Tanja Kokkinis, Communications and Brand Manager
“In 2023 the global omega-3 market size was valued at USD 2.62 billion with an expected compound annual growth rate (CAGR) of 7.9% from 2024 to 2030.
The demand for Omega-3 fatty acids globally is projected to grow significantly over the next few years owing to rising consumer awareness regarding the multiple health benefits of the active ingredient, including lowering blood pressure, managing cholesterol and reducing risks related to heart diseases. As more research is conducted using Omega-3 focusing on newer health categories, such as cancer, support for brain health and the more established health benefits such as inflammation and arthritis, the demand for the supplement is naturally on the rise.
The growing consumer preference for a healthy and nutritious diet is further expected to fuel product demand shortly. Numerous scientific publications have demonstrated the benefits of Omega-3 supplementation and raised awareness for the product’s health benefits among ordinary consumers and not just from within the scientific community. As a result, there is a substantial change in the diet of consumers and everyday foods items are being fortified
with Omega 3 leading to an acceleration in demand for the material globally. Asia is one of the fasted expanding markets within this product category.
Rising pressure on anchovy fisheries to harvest fish oil has increased the demand from non-fish sources, including Evening Primrose Oil, Flax Seed Oil, Walnuts, Algae, and Krill oil. The market is witnessing continuous diversification in its product offerings as consumers are looking for alternatives to traditional fish oils.
The escalating demand for fish oil, which is mainly obtained from anchovy, mackerel, salmon, or herring has led to concerns about overfishing, prompting the industry to seek sustainable alternatives or increase the effectiveness of the products currently on the market. Market players are focusing on R&D to enhance product performance and are creating innovative Self Assembly Drug Delivery Lipid Nanoparticles technology for use in next generation Omega 3 products, which in turn is projected to create lucrative opportunities for the market in the near future.”

Merce
The omega-3 market is set to continue its upward trajectory in 2025, driven by increasing consumer awareness of the essential role omega-3s play in overall health—particularly in supporting heart, brain, and joint health. As demand for high-quality, sustainable omega-3 sources rises, Superba Krill stands out with its unique composition. Unlike traditional fish oils, its omega-3s are in phospholipid form, ensuring superior bioavailability and enhanced absorption, making it a more effective choice for consumers.
Beyond heart health, Superba Krill supports joint function, helps reduce inflammation, and promotes cognitive well-being. Backed by strong scientific research and sourced sustainably, it is well-positioned to meet the growing consumer demand for premium, eco-friendly omega-3 supplements.
Krill oil is an efficient option for women’s health. It is effective in managing dysmenorrhea and emotional PMS symptoms, and for mature women, it may support hormonal balance and reduce inflammation, helping to ease menopausal symptoms. Beyond hormonal health, krill oil’s natural nutrient complex provides essential support for a radiant, healthy glow, contributing to skin health benefits.
Oils from fish, algae and vegetables are a potent source of omega-3, yet processing these unstable raw materials can be complex and challenging. That’s why Nutriswiss AG has developed groundbreaking solutions.

of Research & Development
Omega-3 fatty acids are essential for a healthy diet. And thanks to modern processing techniques, high-quality extracts can be consumed quickly and easily in the form of flavour-neutral food supplements. Food and drinks can also be enriched. However, unsaturated fatty acids oxidise quickly, thus degrading the quality of an oil, while lipids are highly susceptible to environmental pollutants. These can get into the raw oils despite careful sourcing, even with organic products. To negate this, Nutriswiss AG has devised gentle processes for the creation and refinement of taste-neutral, omega-3 oils that retain their valuable, health-promoting properties.
Global demand for marine and plant-based omega-3 products is growing, from dietary supplements, functional foods and beverages to pharmaceuticals, infant formulas, pet food and feed. However, omega-3 oils require a completely different handling process and a higher degree of care than ordinary oils, as their high content of polyunsaturated fatty acids make them unstable and susceptible to oxida-
tion. “Preserving these valuable fatty acids and ensuring that the product remains stable and free from contaminants can be quite challenging,” says Frank Möllering, Head of R&D at the Swiss refinery, Nutriswiss, which specialises in purifying, modifying and refining edible and specialty oils.
While fish are still the dominant source of omega-3, alternatives such as nuts, seeds and oils are becoming more popular, as are microalgae which can be easily cultivated and reproduce quickly. “Fish get their omega-3 from algae, so why not start directly at the source?” says Möllering: “In addition, algae cultures are more environmentally friendly and protect fish stocks. The market is still in the discovery phase but because we’ve been involved in the purification of algae oil for several years now, we process almost as much algae oil as fish oil.”
Regardless of their derivation, all omega-3 oils require careful treatment and processing in order to preserve their essential fatty acids, micronutrients


and vitamins. Nutriswiss therefore relies on a mild, multi-stage refining technology that minimises the formation of process contaminants. At the same time, free fatty acids, plasticisers, pesticides and mineral oil hydrocarbons are largely removed or greatly reduced. In further treatment steps, the company’s experts optimise the sensory quality where necessary in order to produce an omega-3 oil that is entirely neutral in taste and odour.
However, a high proportion of polyunsaturated fatty acids makes oils unstable and susceptible to oxidation. Unlike vegetable oils, the oxidation reaction causes fish oil to develop a strong odour, darken in colour and rapidly become rancid. For sources with an extremely high omega-3 content, the oxidation potential is correspondingly high. To prevent these quality defects, both the raw material and the refined oil must be kept in a protective atmosphere until processed into the end product.
At Nutriswiss, a comprehensive “key figure” profile is initially compiled in its analytical laboratory for each oil received, in order to determine how much “damage” has already occurred. Key factors such as the anisidine value, which measures the secondary degradation products of lipid-compound oxidation, provide information about the history of the raw material.
“Using various process steps, we can remove the secondary oxidation products and reduce the anisidine value to below 10. For a fish oil, this is premium quality. The refined oil is also neutral from a sensory point of view and almost the same light-yellow colour as rapeseed oil,” explains Möllering.
Oxidation products are not the only compounds that have to be removed from a crude oil by refining.

Algae are a high-quality source of DHA and are considered a sustainable alternative to fish oil

Owing to their apolar structure, lipids are especially susceptible to environmental contaminants. Oils from plants often accumulate the insecticides, fungicides and herbicides used in traditional cultivation. In addition, mineral oil saturated hydrocarbons (MOSH) and mineral oil aromatic hydrocarbons (MOAH) may have been transferred to the foodstuff during processing and packaging. Marine animals are particularly at risk: even pesticides that have been banned worldwide for decades, such as polychlorinated biphenyls (PCBs), are still present in waters around the world and accumulate in the fatty tissue of organisms.
For manufacturers who, due to their market positioning, require uncontaminated oils of the highest quality and purity for their formulations, Nutriswiss offers access to a self-developed supply chain. As is already the case with oil plants derived from certified contract cultivation, the Swiss also work with selected partners for marine sources who have committed to the highest standards in terms of catch methods, breeding and transport.
As with almost all sensitive natural raw materials, the challenge is in implementing a gentle purification process to preserve the valuable polyunsaturated fatty acids. To remove the contaminants, high temperatures are required, which in turn favours the formation of other contaminants like trans fatty acids and chlorine-fatty acid esters such as 3-MCPD or glycidyl fatty acid esters. For 3-MCPD, in particular, more stringent regulations have been in force in the EU for infant foods since 2021. And tighter restric-
tions are also being considered for other residues and applications.
Nutriswiss is already prepared for more rigorous legislation. Mild distillation technologies can be used to efficiently remove or significantly reduce impurities in fats and oils. However, it is important that product quality remains stable or is better than that achieved via conventional processes. Nutriswiss has therefore conducted extensive tests to determine the ideal process parameters to meet this goal.
Mild distillation is ideal for sensitive raw materials such as omega-3-rich seed oils, including rapeseed and linseed, as well as algae and other speciality oils. Finding the right blend and quality of oils, especially for infant formula, is a major challenge not only because the fatty acid profile must be optimally balanced, but also because the raw materials must meet the highest quality guidelines and strict specifications for harmful substances. Such oils require customised treatment and a higher degree of care than ordinary oils in order to preserve the omega-3 and omega-6, essential fatty acids as well as tocopherols – better known as vitamin E. As valuable ingredients, tocopherols are welcome not only from a nutritional point of view, but also for product stability and in specific applications.
The mild distillation technology Nutriswiss uses is a gentle, thermal separation process which has already been established in the fish oil sector. Further developed and implemented as one of several purification steps, the plant concept allows contaminants to be removed without generating new ones. It prevents the formation of process contaminants such as 3-MCPD or glycidyl fatty acid esters and, at the same time, removes ortho-phthalates plasticisers and many pesticides. Other pollutants, including MOSH/MOAH, can also be significantly reduced. The gentle process protects micronutrients and can be used to maximise the yield of omega-3 fatty acids.
Developing custom products is one of the great strengths of the Nutriswiss refinery. Before production starts, each process and method is tested in the laboratory, so that all procedures and methods can be adapted to suit individual applications. Afterwards, scale-up - from small quantities of around 500kg batches to several tons - can be done.
The right blend and quality of oils, especially for infant formula, is a major challenge. The fatty acid profile needs to be optimally balanced and all raw materials must meet highest quality guidelines

Nutriswiss also offers advice on sourcing and helps with the selection of the highest quality raw material sources. The company’s portfolio currently includes more than 20 different types of oil and is continuing to grow.
Nutriswiss AG
T. +41 32 387 48 48
F.+41 32 387 48 00
E-mail: info@nutriswiss.ch https://nutriswiss.ch/en/
Fine Foods develops and manufactures contract products for the pharmaceutical, nutraceutical and cosmetics industries.

COMMITTED TO YOUR EXCELLENCE
Coenzyme Q10, also known as ubiquinone, is a critical compound in the human body, playing a vital role in cellular energy production, and also serves as a potent antioxidant. However, the bioavailability of CoQ10 supplements has been a significant challenge, limiting their potential therapeutic benefits.
AUTHOR: Daria Šurić, MPharm, univ.spec.pharm.
Oxidative stress and mitochondrial dysfunction are key contributors to the development of neurodegenerative diseases1. Coenzyme Q10 (CoQ10) is an essential component of the electron transport chain in mitochondria and has been investigated as a potential therapeutic approach for these conditions due to its antioxidant properties and role in energy production1. Additionally, CoQ10 has been studied for its potential benefits in cardiovascular health, fertility, and skeletal muscle function2
One of the primary challenges with CoQ10 supplementation is its poor bioavailability, particularly in targeting specific tissues such as the skeletal and cardiac muscle. This limitation has prompted ongoing research into various formulation strategies to improve the delivery and absorption of CoQ10.
For instance, studies have compared the bioavailability of different CoQ10 formulations, including ubiquinol (the reduced form of CoQ10) and other delivery methods, to address this issue. Furthermore, emerging research has explored the topical application of CoQ10 to the skin and its potential use in ophthalmology, indicating the growing interest in overcoming the bioavailability challenges of this compound2
CoQ10 is produced in the human and animal body. But, despite that, deficiencies may occur. One of the causes might be statine medication usage. Older subjects show an impaired CoQ10 status with lower serum CoQ10 concentration and a higher proportion of the oxidised form. CoQ10 is an essential component of the human electron transport chain in mitochondria, and also an important lipid-
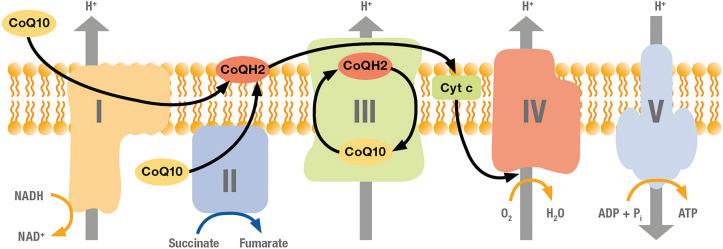
Taken from: Fladerer,J.P.,&Grollitsch,S.(2023).ComparisonofCoenzymeQ10(Ubiquinone)andReducedCoenzymeQ10(Ubiquinol)as Supplement to Prevent Cardiovascular Disease and Reduce Cardiovascular Mortality. Current cardiology reports, 25(12), 1759–1767. https://doi.org/10.1007/s11886-023-01992-6
Mean values are connected by trend lines
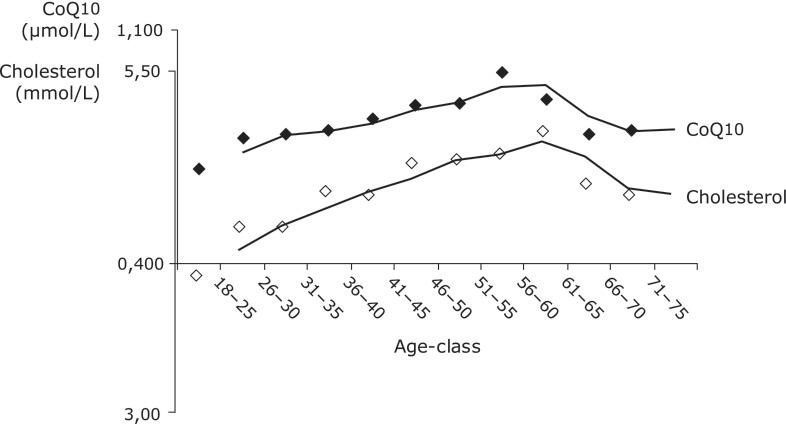
from: Niklowitz, P., Onur, S., Fischer, A., Laudes, M., Palussen, M., Menke, T., & Döring, F. (2016). Coenzyme Q10 serum concentration andredoxstatusinEuropeanadults:influenceofage,sex,andlipoproteinconcentration.Journalofclinicalbiochemistryandnutrition, 58(3),240–245.https://doi.org/10.3164/jcbn.15-73
soluble antioxidant that protects cell membranes and lipoproteins against oxidative damage, preventing peroxidation of the low-density lipoproteins in the blood circulation with additional anti-inflammatory activity.
CoQ10 is a redox molecule occurring in the human body in 2 bioactive states, ubiquinone (CoQ10) as the oxidised state and ubiquinol (CoQH2) as a reduced state. Both redox forms of Coenzyme Q10 are bioactive and important for human health. CoQ10 is essential for cellular adenosine phosphate (ATP) energy production as it shuttles electrons from complexes I and II to complex III of the mitochondrial respiratory chain (Figure 1)3.
CoQH2 is very unstable and under normal conditions oxidised to CoQ10, (II) CoQH2 has to be oxidised to CoQ10 before it can be absorbed in enterocytes, (III) the bioavailability of CoQ10 and CoQH2 mainly depends on crystal dispersion status and carrier oil composition3.
CoQ10 is synthesized by the mevalonate pathway, an essential metabolic pathway, the byproducts of which include cholesterol and other isoprenoids. It is this shared dependence on the mevalonate pathway that brings attention to CoQ10 in cardiovascular conditions4.
CoQ10 is synthesized in almost all human tissues and is presumably involved in age-related alterations and diseases. Women showed lower cholesterol-adjusted CoQ10 levels than men, irrespective of age. As observed in both sexes, the decrease in CoQ10 concentration in older subjects was accompanied by a shift in the redox status in favour of the oxidized form. A strong positive correlation was found between total CoQ10 and cholesterol concentrations. In particular, older subjects were characterized by impaired CoQ10 status due to their lowered serum CoQ10 concentration and concomitant decrea-
se of CoQ10 redox capacity.
CoQ10 is ubiquitously synthesized in almost all cells and membranes of human tissues. CoQ10 is suspected to be involved in age-related alterations of cells and membranes. Due to its two main functions as an electron carrier in mitochondrial bioenergetics and as a lipophilic antioxidant, deficiency in CoQ10 may impair mitochondrial energy production and increase production of reactive oxygen species (ROS), or susceptibility toward them. CoQ10 is associated with several age-related diseases like diabetes, hypercholesterolemia, cardiac insufficiency, and neurodegenerative diseases. The beneficial effects of CoQ10 supplementation are gaining attention. CoQ10 is considered the main antioxidant in lowdensity lipoproteins (LDLs). Lower CoQ10 levels in plasma are related to higher lipid peroxidation.
The study from 2016. was conducted to examine the impact of aging and sex on total and cholesterol-adjusted CoQ10 concentration and redox status in a large cohort of 860 subjects aged 18-82 years.
There was a strong positive correlation between CoQ10 and cholesterol concentrations. Concentrations of both parameters showed similar and parallel changes with age (Figure 2). CoQ10 and LDL-cholesterol concentrations also showed a strong positive correlation. In contrast, HDL-cholesterol level was not significantly related to CoQ10 concentration.
The strong relationship between the total cholesterol or the LDL-cholesterol and CoQ10 concentration respectively in serum was anticipated as virtually all CoQ10 in the circulation is associated with lipoproteins. Furthermore, CoQ10 and cholesterol share in parts a common synthetic pathway. However, the present findings emphasize the necessity for adjustment of CoQ10 concentration to lipid concentration while diagnosing the functional status of CoQ10 in human blood.
It was shown that CoQ10 blood concentrations showed an inverse U-shaped course during adulthood. In old people, the antioxidative properties of CoQ10 in circulation may be impaired, as reflected by a decrease in CoQ10 concentrations, accompanied by a shift in redox status in favour of the oxidized proportion. This shift in redox status was seen in both sexes; however, women irrespective of age had lower cholesterol-adjusted CoQ10 levels in comparison to men. Therefore, women may either be more susceptible to oxidative damage or men may have an increased demand for antioxidant capacity.
There is a clear negative correlation between the cholesterol-adjusted CoQ10 level and its oxidized proportion of CoQ10. Thus, the decrease of CoQ10 levels in old age associated with a loss of antioxidant capacity is not limited to human tissues or cell organelles but applies also to lipoprotein protection in the blood. The concurrent occurrence of low cholesterol-adjusted concentrations and high oxidized proportions of CoQ10 increases the susceptibility to oxidative stress in old age, which may be overcome by oral supplementation5
Maintenance of CoQ10 levels in plasma is import-
ant in preventing LDL oxidation, thereby reducing cardiovascular disease risk and preventing muscle deterioration during aging. For this reason, supplementation with CoQ10 can be recommended in cases of deficiency to restore, at least, the antioxidant capacity and to avoid LDL oxidation. However, studies of bioavailability show that the response of individuals to CoQ10 supplementation is very variable. For this reason, considerable research has been carried out to increase its bioavailability including the use of different types of liposomes or new surfactants.
The bioavailability of supplements with CoQ10 in humans seems to depend on the excipients of formulations and the physiological characteristics of the individuals. Both CoQ10 and cholesterol concentrations were positively correlated with BMI. BMI and the proportion of the oxidized form of CoQ10 were also positively correlated, which indicates increased oxidative stress in obese people6
Cardiovascular research, fertility and skeletal muscle health remain major fields of application of Coenzyme Q10 (Figure 3). Novel applications, particularly topical applications, are gaining considerable interest. In this respect, bioavailability represents a major challenge and the innovation in formulating is
3 Areas of CoQ10 application in human studies for improving human health.
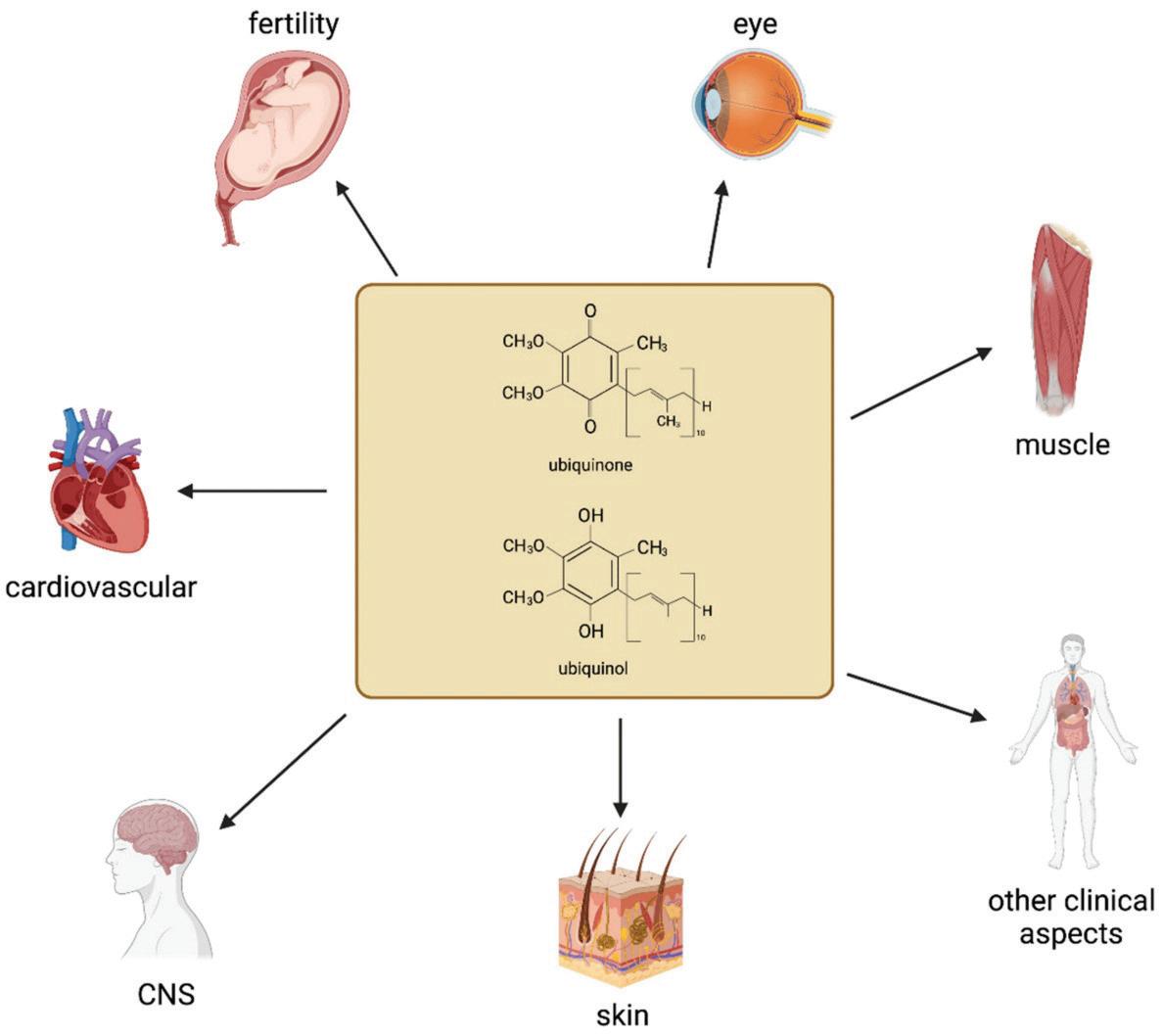
CNS = Central Nervous System. Taken from: Cirilli,I.;Damiani,E.;Dludla,P.V.;Hargreaves,I.;Marcheggiani,F.;Millichap,L.E.;Orlando,P.;Silvestri,S.;Tiano,L. RoleofCoenzymeQ10inHealthandDisease:AnUpdateontheLast10Years(2010–2020).Antioxidants2021,10,1325. https://doi.org/10.3390/antiox10081325

gaining critical importance.
More than six decades after its discovery, CoQ10 research continues to drive significant interest in the scientific community, particularly in the second decade of the new millennium. This enduring attention is driven by its diverse mechanisms of action and broad applications in human health. The beneficial effects of CoQ are largely attributed to its well-established bioenergetic and antioxidant properties, though emerging studies increasingly emphasize its role in redox signalling and mitochondrial function modulation. However, its limited bioavailability remains a key challenge. To address this, researchers are actively exploring novel formulation strategies. Notably, topical applications of ubiquinol, particularly in dermatology and ophthalmology, are gaining attention as promising therapeutic approaches.
References:
1 Bliznakov, E. G., & Bhagavan, H. N. (2002). Oxidative stress, bioenergetics and neurodegenerative diseases. In Journal of Neurochemistry (Vol. 81, p. 60). Wiley. https://doi.org/10.1046/j.1471-4159.81.s1.20_16.x
2 Cirilli, I., Damiani, E., Dludla, P. V., Hargreaves, I. P., Marcheggiani, F., Millichap, L., Orlando, P., Silvestri, S., & Tiano, L. (2021). Role of Coenzyme Q10 in Health and Disease: An Update on the Last 10 Years (2010–2020) [Review of Role of Coenzyme Q10 in Health and Disease: An Update on the Last 10 Years (2010–2020)]. Antioxidants, 10(8), 1325. Multidisciplinary Digital Publishing Institute. https://doi.org/10.3390/antiox10081325
3 Fladerer, J. P., & Grollitsch, S. (2023). Comparison of Coenzyme Q10 (Ubiquinone) and Reduced Coenzyme Q10 (Ubiquinol) as Supplement to Prevent Cardiovascular Disease and Reduce Cardiovascular Mortality. Current cardiology reports, 25(12), 1759–1767. https://doi.org/10.1007/s11886-023-01992-6
4 Raizner, A. E., & Quiñones, M. A. (2021). Coenzyme Q10 for Patients With Cardiovascular Disease: JACC Focus Seminar. Journal of the American College of Cardiology, 77(5), 609–619. https://doi.org/10.1016/j.jacc.2020.12.009
5 Niklowitz, P., Onur, S., Fischer, A., Laudes, M., Palussen, M., Menke, T., & Döring, F. (2016). Coenzyme Q10 serum concentration and redox status in European adults: influence of age, sex, and lipoprotein concentration. Journal of clinical biochemistry and nutrition, 58(3), 240–245. https://doi.org/10.3164/jcbn.15-73
6 López-Lluch, G., Del Pozo-Cruz, J., Sánchez-Cuesta, A., Cortés-Rodríguez, A. B., & Navas, P. (2019). Bioavailability of coenzyme Q10 supplements depends on carrier lipids and solubilization. Nutrition (Burbank, Los Angeles County, Calif.), 57, 133–140. https://doi.org/10.1016/j.nut.2018.05.020
7 Cirilli, I.; Damiani, E.; Dludla, P.V.; Hargreaves, I.; Marcheggiani, F.; Millichap, L.E.; Orlando, P.; Silvestri, S.; Tiano, L. Role of Coenzyme Q10 in Health and Disease: An Update on the Last 10 Years (2010–2020). Antioxidants 2021, 10, 1325. https://doi.org/10.3390/antiox10081325
CoQ10 supplementation at 200 mg/day, alone or combined with 400 IU/day of Vitamin E, shows beneficial effects on insulin sensitivity and androgen levels in women with PCOS, while combined supplementation significantly improves SHBG concentrations, suggesting that a dual approach may be more effective.
The following study investigates the effects of Coenzyme Q10 (CoQ10) and Vitamin E supplementation on metabolic and hormonal parameters in women with polycystic ovary syndrome (PCOS). This randomised, double-blind, placebocontrolled trial included 86 women who received either CoQ10 (200 mg/day) and/or Vitamin E (400 IU/day), a combination of both, or a placebo for eight weeks.

The results indicate that CoQ10, whether alone or combined with Vitamin E, significantly reduced fasting blood sugar (FBS) levels and improved insulin resistance (HOMA-IR). However, Vitamin E alone did not have a significant effect on glucose metabolism. In terms of hormonal outcomes, supplementation with CoQ10 (200 mg/day) and/or Vitamin E (400 IU/day) led to a notable decrease in total testosterone levels and the free androgen index (FAI). Among the groups, only the combination of CoQ10 and Vitamin E increased sex hormone-binding globulin (SHBG) levels, suggesting a potential synergistic effect.
The study attributes these improvements to CoQ10’s role in mitochondrial energy production, antioxidant activity, and insulin regulation, which may help mitigate insulin resistance and hyperandrogenism in PCOS. Vitamin E, known for its antioxidant properties, may contribute by reducing oxidative stress, a key factor in the pathophysiology of PCOS. Additionally, the findings highlight that improvements in glucose metabolism were directly associated with reductions in androgen levels, reinforcing the link between insulin resistance and hormonal imbalances in PCOS.
In conclusion, CoQ10 supplementation at 200 mg/day, alone or combined with 400 IU/day of Vitamin E, demonstrates beneficial effects on insulin sensitivity and androgen levels in women with PCOS. However, only the combined supplementation significantly improved SHBG concentrations, suggesting that a dual approach may be more effective. These findings underscore the potential of nutritional and metabolic interventions as adjunct therapies for managing PCOS.
Reference:
Izadi, A., Ebrahimi, S., Shirazi, S., Taghizadeh, S., Parizad, M., Farzadi, L., & Gargari, B. P. (2019). Hormonal and Metabolic Effects of Coenzyme Q10 and/or Vitamin E in Patients With Polycystic Ovary Syndrome. The Journal of clinical endocrinology and metabolism, 104(2), 319–327. https://doi.org/10.1210/jc.2018-01221
Gencor has announced the publication of its recent human clinical study investigating Tesnor®, its proprietary blend of cacao (Theobroma cacao) seed and pomegranate (Punicagranatum) peel for aging male sexual function.


Men typically experience an age-related decline in testosterone, by approximately 0.4 to 2% per year after the age of 301. Testosterone is the primary androgen responsible for the growth and development of the male reproductive system, helping to maintain key male sexual characteristics such as sexual function, desire, performance, vigor, a healthy sperm profile, bone and muscle mass, metabolic balance, and psychological well-being2
Tesnor® has been clinically studied for its ability to increase serum testosterone levels in both young3 and aging males4; therefore, this study aimed to examine the sexual function of male volunteers aged 40 to 70 with serum total testosterone ≥ 300 ng/dL.
The 84-day randomized, double-blind, placebocontrolled trial included 120 men who were randomly assigned to receive either 400 mg of Tesnor® or matching placebo each morning. The results indicated that Tesnor® improved sexual function, libido, psychological well-being, muscle strength, and overall well-being in aging males.
“The cacao seed has long been used in traditional Indian medicine to promote sexual health, while pomegranate is recognized as a powerful antioxidant that supports blood flow and sexual function. Tesnor, our proprietary blend, is clinically proven to boost testosterone levels,” said R.V. Venkatesh, CoFounder and Managing Director at Gencor. “The results of this study further demonstrate Tesnor's effectiveness in enhancing men's health just beyond serum testosterone levels to showing improved sexual health in aging males.”
Tesnor® is a standardized, IP-protected, plant-derived testosterone booster suitable for multiple applications including capsules, tablets, ready-to-mix (RTM) powders, softgels, and gummies.
To review the additional published clinical data on Tesnor®, visit tesnor.us or GencorPacific.com
References:
1 https://pubmed.ncbi.nlm.nih.gov/26834840/
2 https://joe.bioscientifica.com/view/journals/joe/217/3/R25.xml
3 https://cdn.prod.website-files.com/5f85d0c81b6f5da2719b6b03/6540237ae02f41f305287f2c_Tesnor%20Study_Increas-
es%20Serum%20Testosterone%20Level%20in%20Young%20 Males.pdf
4 https://cdn.prod.website-files.com/5f85d0c81b6f5da2719b6b03/65402369d6509fe917cf8138_Tesnor%20Study_mitigates%20aging%20males%20symptoms.pdf

Gencor has announced the publication of its recent human clinical study investigating its palmitoylethanolamide (PEA) ingredient, Levagen®+, to be a safe, innovative, and effective supplement to ease acute menstrual discomfort.
Menstrual pain, or primary dysmenorrhea (menstrual pain without any underlying pathology), is the most common gynecological condition among women of reproductive age. It is estimated to be the leading cause of chronic pelvic pain globally (World Health Organization). The prevalence rate ranges from 45 to 95% and is linked to restrictions in daily activities, work and school absenteeism, and personal distress.
While the primary mechanisms of dysmenorrhea are not fully understood, the main cause of the pain-


ful symptoms is attributed to the overproduction of uterine prostaglandins, particularly prostaglandin F2-α (PGF2α) and prostaglandin E2 (PGE2), which peak during the early days of menstruation. Besides their regulatory effects on inflammation, pain, and body temperature, prostaglandin production is also thought to contribute to secondary symptoms of menstrual pain, such as diarrhea, nausea, and vomiting. Moreover, women experiencing dysmenorrhea may show elevated levels of pro-inflammatory mediators and an increased body temperature.
Preclinical research suggests that PEA’s primary mechanism of action is to provide relief from occasional discomfort and support a balanced inflammatory response, which may be through the activation of peroxisome proliferator-activated receptor-α (PPAR α). Therefore, this study aimed to assess the effectiveness of PEA in alleviating acute menstrual pain in otherwise healthy women.
The randomized, crossover, double-blind, placebo-controlled trial included 80 menstruating females aged 18 years and older self-reporting mild to moderate menstruating cramp pain. Each randomized bottle of the trial product contained either 350mg of Levagen+ or the placebo (350mg of microcrystalline cellulose). Pain scores were recorded on the numerical pain rating scale (NRS) every 30 minutes for up to 4 hours. The findings indicate Levagen+ significantly reduced pain scores compared to placebo at 1, 1.5, 2, and 2.5 hours post-dose, with a pain reduction of approximately 25 percent by 2.5 hours compared to the placebo group. This study demonstrates that Levagen®+ was an effective strategy to reduce acute menstrual pain in healthy women.
“Despite its negative effect on daily life and common occurrence, menstrual pain is often perceived as a ‘normal’ facet of a woman’s menstrual cycle,” said R.V. Venkatesh, Co-Founder and Managing Director at Gencor. “The results of this study show that Levagen®+ can be an effective and quick-acting supplement to support women during their monthly cycles. We are excited to continue to explore how Levagen®+ can support female health.”
To review the published clinical data and for more information on Levagen®+, visit www.levagenplus.com or GencorPacific.com

The world’s nutraceutical event promises visitors the same Vitafoods Europe experience they know and love in a fantastic new location with even more opportunities to connect, innovate, and explore the ever-evolving nutraceutical landscape.

Mark your calendars, Vitafoods Europe is back for 2025 and is set for its biggest edition yet! But this time, it’s making a move to the vibrant city of Barcelona. Taking place at Fira Barcelona, Gran Via between 20-22 May and boasting a record 75,000m2 of exhibition space, the world’s nutraceutical event promises visitors the same Vitafoods Europe experience they know and love in a fantastic new location with even more opportunities to connect, innovate, and explore the ever-evolving nutraceutical landscape. Want to know what’s in store for the 2025 show? Read on to find out!
Hola Barcelona!
Perhaps one of the most exciting updates for 2025 is Vitafoods Europe's move to the city of Barcelona – a decision that reflects the booming nutrition industry in Spain, one of the top 5 countries in the European nutraceuticals market.1 With world-class accommodation and excellent transport links, the move is set to elevate the event to new heights by enabling more visitors and exhibitors to attend –creating ample opportunities for businesses to network and be inspired. What’s more, Barcelona’s rich culture, thriving sports scene, and delicious cuisine make it an exciting destination for global visitors. And with the introduction of Vitafoods Europe, this bustling city is set establish itself as the ultimate hub for the global nutraceutical community.

Whether you’re a Vitafoods Europe veteran or newbie, at this year’s event you can expect an expanded lineup of content and increased networking opportunities alongside the return of all-time favourite features, newly designed to address the needs of the entire nutraceutical supply chain. Here’s what’s on the agenda for 2025:
Brand-new for 2025, the Nutraceuticals Showcase Theatre will feature dedicated presentations covering sectors across the industry. With over half of event visitors interested in finished products, the Nutraceuticals Showcase Theatre provides a great opportunity to get a 360° view of nutraceutical product innovation – with dedicated sessions covering contract manufacturing, finished products and beyond.
Another feature making its debut in 2025 is the Future of Nutrition Lunch and Learn. The session’s theme – ‘hack the mass-market: creating gamechanging nutraceutical products’ – promises a unique chance to learn from influential industry leaders and discover cutting-edge solutions before they hit the market. Expect exclusive, actionable insights from trailblazers who have transformed niche products into mainstream successes and redefined traditional consumer categories along the way – it’s one not to miss!
Women’s health is an increasingly hot topic dominating the nutraceutical industry, with growing consumer awareness about the role of supplementation in supporting hormonal health, fertility, pregnancy, and more. It’s therefore no surprise that women’s health will be a big focus at Vitafoods Europe 2025, as highlighted by the all-new Women’s Health Spotlight. Held at the Conference Theatre on 22 May, the spotlight will provide science-backed insights into challenges across all stages of a woman’s life. The session will explore the latest breakthroughs in menopause management, addressing both physical and emotional health during this pivotal life stage. It will also feature explorations of beauty-from-within solutions for hair, nail, and skin health – highlighting how nutraceuticals are rising to meet women’s growing demand for holistic wellbeing.
Your
Vitafoods.eu.com





For those looking for the signature Vitafoods experience, a fan favourite returns in the form of the Vitafoods Europe Conference. Between 20-21 May, this two-day programme will bring together top R&D, product development, and innovation professionals to share insights into groundbreaking strategies revolutionizing the industry. Visit the Vitafoods Conference Theatre for expert presentations, panel discussions, and scientific case studies on four key themes: Sports Nutrition and Active Lifestyle; Healthy Gut, Healthy Life; Cognitive and Emotional Health; and Lifelong Health.
A cornerstone of the event, the Vitafoods Insights Theatre also returns, presenting thoughtprovoking discussions on market trends and consumer insights, sustainability, regulatory updates, and supply chain developments. Sessions will explore everything from tech-driven innovation, to branding and marketing strategies, as well as tackle key topics such as the importance of diversity and inclusion in the industry.
While you’re gaining insights at the show, why not also take the opportunity to gain connections? This year’s event will offer attendees more opportunities than ever to build meaningful connections with experts and suppliers across the industry. The Women’s Networking Breakfast on 21 May, sponsored by Kerry, offers a powerful platform for professional growth and collaboration through inspiring panel discussion and networking breakout sessions. Whether you are an entrepreneur, business leader, or an advocate for diversity in the industry, this is a mustattend session for all.
Additionally, the new Networking Brunch on 22 May, will explore the future of personalised nutrition and biohacking. Featuring industry pioneers, the event will cover innovations in AI, machine learning, epigenetics, and diagnostic technologies. Experts will also address sustainability and affordability challenges in bringing personalised health solutions to the mass market.
2025’s event will also see the debut of the Vitafoods Europe Innovation Awards, celebrating excellence in nutraceutical innovation. Categories will recognise achievements in sports nutrition and active lifestyle; immune and gut health; cognitive and emotional health; healthy ageing; and more. Exhibitors have until 10 March to submit their entries before a shortlist of finalists are invited to present pitches live to an expert judging panel in Barcelona. Winners will be announced at an exclusive ceremony on the evening of 20 May at Fira Barcelona, Gran Via, with winning companies receiving increased visibility throughout the 2025 event.
With 75,000m2 of exhibition space to play with this year – Vitafoods Europe’s largest ever show floor –visitors have the opportunity to lose themselves in even more features and insights at this year’s show. One highlight of 2025’s extended show floor is the significantly expanded Finished Products Area, where exhibitors will showcase the latest high-quality, market-ready innovations. With more exhibitors, more visitors and more features, Vitafoods Europe promises extra value for businesses across the supply chain and enhanced insights which have never been seen before.
Vitafoods Europe 2025 is set to be the most exciting and impactful edition yet. Whether you’re an industry leader, a passionate innovator, or inquisitive about insights, this event is your gateway to the future of nutrition.
Registrations for Vitafoods Europe 2025 are open. Book your ticket by 10 March to secure your pass at a limited-time rate of €99: www.vitafoods.eu.com
Reference:
1 Grand View Research, ‘Nutraceuticals Market Report’, 2023


lnflamrnation in the body can result from physical or athletic exertion. And while a crucial and useful biologica I proces�, such chronic respon�es can damage tissue and cause injuries, leading to various conditions. Because it's composed exclusively of THC s - the major active metabolites of curcumin - C3 Reduct ® delivers anti-inflammatory properties that hold significant promises in protecting and soothing joints and muscles.





Developing effective nutraceutical products for cellulite reduction and skin firmness requires a strategic approach, incorporating evidence-based ingredients, optimal dosages, appropriate delivery mechanisms, and compliance with regulatory standards. This guide provides a structured framework for formulating such products.
Objective: Determine if the product targets cellulite reduction, skin elasticity, hydration, or overall skin health.
Target audience: Identify key demographics such as women experiencing cellulite due to hormonal changes, aging individuals, or fitness-conscious consumers.
Focus on scientifically-backed ingredients that address cellulite and skin firmness from within.
Collagen and protein-based ingredients
Hydrolyzed collagen peptides: Supports skin elasticity and dermal thickness.
EXAMPLE: Verisol® by Gelita, clinically shown to improve skin elasticity and reduce wrinkles.
Elastin peptides: Enhances skin resilience and elasticity.
EXAMPLE: Peptan® Elastin for structural skin benefits.
Vascular and circulation-enhancing agents
Horse chestnut extract (Aescin): Improves blood flow and reduces fluid retention.
Grape seed extract (proanthocyanidins): Enhances microcirculation and supports skin firmness.
EXAMPLE: Vintera™ from Elementia.
Anti-inflammatory and antioxidant compounds
Vitamin C: Essential for collagen synthesis and skin firmness.
Astaxanthin: Protects against oxidative stress and supports skin elasticity.

EXAMPLE: AstaReal®, backed by clinical studies.
Curcumin (tetrahydrocurcuminoids): Reduces inflammation and improves skin tone.
EXAMPLE: Curcumin C3 Reduct® by Sabinsa.
Lipolytic and fat metabolism enhancers
Caffeine: Stimulates lipolysis and reduces localized fat deposits.
Example: Ingredient Pharm microencapsulated caffeine form for sustained release.
Conjugated linoleic acid (CLA): Aids in fat metabolism and body composition improvement. Example: DurOmega® CLA from InnoBio.
L-Carnitine: Supports fatty acid oxidation, potentially reducing adipocyte accumulation.
Hormonal and cortisol modulating ingredients
Ashwagandha (Withania somnifera): Helps regulate cortisol, which may influence fat storage.
EXAMPLE: Shaganda® by Sabinsa, a high-potency extract that complies with US Monograph.
Rhodiola rosea: Supports stress resilience and hormonal balance.
Saffron extract: Affects serotonin levels and mood, indirectly impacting fat accumulation.
Example: Affron® by Pharmactive, backed up with clinical studies.
3. ESTABLISH EFFECTIVE DOSAGES
Dosages should align with scientific research:
Collagen peptides: 2.5-10 g/day.
Grape seed extract: 150-300 mg/day.
Caffeine: 100-200 mg/day (consider sustained-release forms).
CLA: 3-6 g/day.
Ashwagandha: root extract 300-600 mg/day. Saffron extract: stigma extract 30 mg/day.
4. CHOOSE A DELIVERY FORM
Selecting the appropriate format enhances compliance and efficacy:
• Capsules/tablets: Precise dosing and ease of use.
• Powders: Ideal for collagen-based formulations and multi-ingredient blends.
• Beverages: Functional drinks targeting hydration and cellulite reduction.
• Functional gummies: Increasingly popular for beauty-from-within applications.
5. INCORPORATE SYNERGISTIC BLENDS
Combine collagen peptides with vitamin C to enhance collagen synthesis.
Pair caffeine with L-carnitine for improved lipolysis. Use rhodiola and ashwagandha together for stress modulation and hormonal balance.
Integrate grape seed extract with horse chestnut for enhanced circulation benefits.
TESTING
Ensure ingredient stability and efficacy: Check for oxidation in polyphenol-rich formulations. Validate sustained-release caffeine for long-lasting effects.
Assess the impact of environmental factors on collagen peptide stability.
7. REGULATORY COMPLIANCE
Labeling: Follow region-specific regulations (FDA in the U.S., EFSA in Europe).
GRAS status: Ensure ingredients are Generally Recognized as Safe.
In some European countries on-hold botanical claims can be used, check the legislation on a country's level.
EFSA-approved claims:
Vitamin C contributes to normal collagen formation. Zinc contributes to the maintenance of normal skin. Magnesium contributes to normal energy-yielding metabolism and protein synthesis.
8. CONDUCT EFFICACY STUDIES
Clinical trials or pilot studies can substantiate claims for cellulite reduction and skin firmness. Consumer trials can provide real-world feedback on product effectiveness.
9. MARKET POSITIONING
Claims: "Supports Skin Firmness," "Reduces Cellulite Appearance," "Enhances Collagen Production."
Target messaging: Address concerns of beauty-conscious consumers, aging individuals, and wellness enthusiasts.
B2B communication: Provide scientific substantiation for formulators and brand owners.
10. CONTINUOUS IMPROVEMENT
Stay updated on new research regarding skin health and cellulite mechanisms.
Monitor customer feedback for potential reformulations.
Explore emerging bioactive ingredients for enhanced efficacy.
Conclusion
By adhering to this structured formulation approach, companies can develop safe, effective, and marketable products, targeting cellulite reduction and skin firmness from the inside out.
Darmell Ltd.
20+ years of experience in developing new concepts for food supplements
Mob: + 385 91 68 12 444 darmell@protonmail.com www.dar-mell.com


Exosomes are revolutionizing the cosmetic industry by offering a natural, biotechnology-driven approach to skin regeneration, anti-aging, and protection. These nanosized extracellular vesicles facilitate targeted delivery of bioactive molecules, enhancing collagen synthesis, reducing inflammation, and improving skin barrier function. As research advances, exosome-based skincare is poised to become a key innovation in next-generation beauty formulations.

AUTHOR:
Gordana Gorinšek, MSc in Phytomedicine, Expert Cosmetic Safety
Assessor, Expert Regulatory Affairs Consultant
Exosomes have emerged as a revolutionary component in cosmetic science due to their role in cell communication, regeneration, and anti-aging processes. These nanosized extracellular vesicles facilitate the transfer of bioactive molecules such as proteins, lipids, and RNAs, influencing skin cell function and repair mechanisms. Their primary function is intercellular communication, transferring molecular signals that regulate cellular behaviour.
What are exosomes and how are they created?
Exosomes are not cells but are produced by the cells of every living organism, floating freely in the extracellular space. These extremely small vesicles, similar to bubbles, measure between 30 and 150 nanometers (nm) in diameter and are secreted by various cell types, including stem cells, fibroblasts, and keratinocytes. They possess bilayer membranes and transport essential biomolecules such as proteins, carbohydrates, lipids, and nucleic acids. Particularly significant are exosomes derived from stem cells due to their potential for continuous mitosis and their undifferentiated state.
Exosomes are formed through a process called endocytosis, in which the cell membrane invaginates inward, creating vesicles known as endosomes that contain various biomolecules such as DNA, RNA, growth factors, cytokines, and membrane proteins. These endosomes eventually fuse with the plasma membrane through exocytosis, releasing their contents into the extracellular space. At this point, they become extracellular vesicles known as exosomes. Once in circulation, exosomes bind to proteins on the surface of target cells, where they are internalized through endocytosis, direct membrane fusion, or receptor-ligand interactions, allowing for the delivery of their bioactive cargo.
In recent years, their role in dermatology and cosmetic formulations has gained attention due to their potential in skin rejuvenation, wound healing, and anti-inflammatory responses.
Mechanism of action in cosmetics
Exosomes influence skin well-being through several key mechanisms:
1. Cellular communication and regeneration: Exosomes contain growth factors (e.g., epidermal growth factor, fibroblast growth factor), cytokines, and microRNAs that enhance fibroblast activity, collagen synthesis, and skin repair.

2. Preservation of youthful appearance: By promoting the synthesis of collagen and elastin, exosomes can help reduce fine lines and wrinkles, improving skin elasticity and firmness.
3. Anti-anflammatory and antioxidant properties: Exosomes derived from stem cells can modulate inflammatory responses, reducing oxidative stress and preventing premature aging caused by environmental factors.
4. Skin repair and barrier function: They can accelerate skin regeneration by stimulating keratinocyte proliferation and migration, enhancing the skin barrier and hydration levels.
5. Anti-pollution effects: Exosomes can bind heavy metals from water and air, helping protect skin and hair from urban pollution and smog.
The primary sources of exosomes used in skincare formulations include:
• Animal cells: stem cells, B cells, dendritic cells, and mast cells.
• Bodily fluids: plasma, serum, urine, cerebrospinal fluid, and interstitial fluid.
• Plant-derived exosomes: similar to those from animal cells.
• Microorganisms-derived exosomes: byproducts of microorganisms.
In the cosmetic industry, exosomes derived from plants and microorganisms have primacy over human exosomes. Exosomes used in cosmetics are typically found in the form of lyophilized powders or aqueous solutions and are approximately 300 times smaller than the average pore size, allowing excellent skin absorption and penetration.
1. Cell communication and regeneration: Exosomes derived from stem cells can promote skin regeneration, repair, and rejuvenation by delivering growth factors and cytokines that enhance cellular proliferation and skin repair.
2. Preservation of youthful appearance: Exosome formulations can enhance collagen synthesis, improve skin elasticity, and reduce wrinkles by stimulating the production of new skin cells.
3. Targeted delivery: Exosomes can serve as natural delivery systems for active ingredients in cosmetic formulations, improving absorption and effectiveness compared to traditional delivery methods.
4. Anti-inflammatory effects: Exosomes possess anti-inflammatory properties, helping to soothe irritated skin and reduce redness or puffiness, making them ideal for sensitive skin formulations.
5. Skin barrier function: By promoting the production of skin barrier proteins, exosomes can enhance skin hydration and protection against environmental stressors.
The integration of exosomes into cosmetic formulations represents a biotechnological revolution due to several key factors:
• Innovative ingredients: Exosomes provide a novel source of bioactive compounds that can enhance the effectiveness of cosmetic products, enabling the development of more advanced formulations.
• Research and development: As exosome science is still in its early stages, there is vast potential for further research and development in this field. Ongoing studies are exploring specific applications of exosomes in cosmetic products.
• Regulatory considerations: The use of exosomes in cosmetics may raise regulatory concerns. Let's not forget that exosomes are nano-particles in size. Companies must ensure that their products are safe and effective, which may involve extensive testing and adherence to existing cosmetic regulations. This concerns not only cosmetic producers, but cosmetic raw material producers also.
• Consumer awareness: As consumers become more informed about skincare ingredients, the appeal of innovative solutions like exosomes may drive demand for products utilizing these advanced compounds.
While exosome-based cosmetics offer promising benefits, several challenges must be addressed:
• Safety and purity: Ensuring that exosomes are free from contaminants and pathogens is crucial for consumer safety.
• Standardization: The cosmetic industry currently lacks standardized protocols for exosome isolation, dosage, and formulation. Standardization rests on the level of internal protocols of raw material producers.
• Long-term efficacy: Although initial studies are promising, further clinical trials are necessary to validate the long-term benefits and possible side effects.
Exosomes represent a groundbreaking advancement in cosmetic formulations, offering regenerative, anti-aging, and protective benefits for the skin. However, their widespread adoption requires further research, regulatory guidelines if and when necessary, and advancements in production techniques to ensure efficacy and safety. As cosmetic science progresses, exosome-based skincare may become a key component of next-generation cosmetic products. They certainly have great potential for use in preparative cosmetics.

Aromatični kutak Ltd. Brune Bušića 21 Zagreb, Croatia https://pif.com.hr/ info@aromaticnikutak.hr T. + 385 98 1750 934

Portable lab-on-a-chip device uses cutting-edge science to enable consumers to understand their skin’s aging trajectory and gain insights into the skincare that works best for their needs.

At the CES® 2025, L'Oréal Groupe unveiled
L’Oréal Cell BioPrint, a tabletop hardware device that provides personalized skin analysis in just five minutes, using advanced proteomics –the study of how protein composition in the human body affects skin aging.
L’Oréal Cell BioPrint is made possible by L'Oréal's Longevity Integrative Science™, a groundbreaking approach that reveals how mechanisms in the human body can affect skin’s appearance, and through an exclusive partnership with Korean startup NanoEnTek.
L’Oréal Cell BioPrint produces a personalized skin assessment in minutes including:
• Skin's biological age: How fast is skin aging?
L’Oréal Cell BioPrint can calculate skin's age and provide personalized advice on how to slow down the appearance of aging.
• Ingredient responsiveness: Will certain active ingredients work on one’s skin? L’Oréal Cell BioPrint minimizes guesswork by helping to predict responsiveness to certain key ingredients such as retinol.
• Shifting from reactive to proactive skincare: Is one’s skin prone to dark spots or enlarged pores?
L’Oréal Cell BioPrint can help predict potential cosmetic issues before they become visible, enabling users to take proactive steps to help protect the beauty of their skin.
"At L'Oréal, we're always looking toward the future of beauty, blending cutting-edge discoveries with our long-standing beauty expertise. With skin being the largest organ, and a key part of people’s wellbeing, we are thrilled to unveil Cell BioPrint, an exclusive microfluidic lab-on-a-chip technology coupled with our century-long skin science leadership. With the Cell BioPrint device, we offer people the ability to discover deeper insights about their skin through specific biomarkers and to proactively address the beauty and longevity of their skin,” said Barbara Lavernos, Deputy CEO in charge of Research, Innovation and Technology at L’Oréal Groupe.
In pursuit of increased personalization in skincare
The growth of the global skincare market, which is
projected to reach $125 billion in 20241, is driven by consumers who are continuously seeking more information about, and more efficacious products for, their unique skin. According to a recent US survey of 2,000 skincare users, nearly 80% reported relying on trial and error to determine what worked for them, with the average person reporting trying seven different cleansers before finding one they love2
The advanced science in L’Oréal Cell BioPrint is now being applied to skin intelligence thanks to decades of knowledge-building and innovation by L'Oréal’s Advanced Research team, which identified for the first time, unique biomarkers in the skin that can indicate key components of healthy-looking skin and longevity3.
The L’Oréal Cell BioPrint device also features NanoEntek’s exclusive microfluidic lab-on-a-chip technology, which leverages some of NanoEnTek’s 100+ patents to measure the presence of L’Oréal‘s groundbreaking protein biomarkers in five minutes. It works through a simple, non-invasive process comprised of the following steps:
1. Put a facial tape strip on one’s cheek, then place into buffer solution.
2. Load the solution into the L’Oréal Cell BioPrint cartridge and insert it into the machine for analysis.
3. While L’Oréal Cell BioPrint processes the sample, the Skin Connect device takes several images of one’s face and a short questionnaire about skin concerns and aging is completed.
L’Oréal Cell BioPrint is scheduled to pilot with a L'Oréal brand in Asia later in 2025.
References:
1 BMS T2024l.
2 Conducted by OnePoll in conjunction with CeraVe.
3 ”Clinical vs. chronological skin age: exploring determinants and stratum corneum protein markers of differential skin ageing in 351 healthy women,” by A. Foucher, S. Nouveau, V. Piffaut, S. Marque, L. Aguilar & N. Cavusoglu, Nature, October 9, 2024.
A deciduous, thorny shrub or small tree from the Rosaceae family, hawthorn is known for its medicinal properties and long history of use in herbal medicine. This resilient plant contains flavonoids and other bioactive compounds that contribute to its therapeutic effects, particularly in improving heart function and circulation.
Crataegus oxyacantha , commonly known as Hawthorn, is a deciduous, thorny shrub or small tree belonging to the Rosaceae family. It typically grows 5-10 meters in height and features deeply lobed, dark green leaves. The plant produces small, fragrant white or pink flowers in clusters during late spring. These flowers develop into bright red, berry-like fruits known as haws, which persist into winter.
Hawthorn is recognized for its dense, spiny branches, which provide shelter and food for wildlife. The tree thrives in temperate regions, particularly in Europe, North America, and Asia.
Hawthorn is commonly found in hedgerows, woodlands, and open fields. It thrives in well-drained, loamy soils and prefers full sun to partial shade. This hardy plant can tolerate a wide range of environmental conditions, including drought and cold temperatures, making it a resilient species in various climates.
Hawthorn is often used in ornamental landscaping, as well as for conservation purposes, such as soil stabilization and wildlife habitat enhancement.
Hawthorn has been traditionally used for its cardiovascular benefits. The plant contains flavonoids and other bioactive compounds that contribute to its therapeutic effects, particularly in improving heart function and circulation.

Traditional medicinal uses
Hawthorn has been used in herbal medicine for centuries. Common applications include: supporting heart health and regulating blood pressure, treating mild heart failure, angina, and arrhythmias, aiding digestion and relieving mild gastrointestinal discomfort and acting as a mild sedative to reduce stress and anxiety.
Constituents
Flavonoids (e.g., quercetin, rutin)
• Procyanidins
• Triterpenic acids
• Phenolic acids
• Amine compounds (e.g., trimethylamine)
Vitamin C
Action and application
The bioactive compounds in Hawthorn are known for their cardioprotective effects. They enhance coronary artery blood flow, improve oxygen utilization in the heart, and help maintain normal blood pressure levels. Hawthorn extracts are widely used in modern herbal medicine as a natural remedy for heart conditions.
Additionally, Hawthorn’s antioxidant properties protect cells from oxidative stress, while its mild sedative effects make it beneficial for anxiety and nervous tension.
While generally safe, Hawthorn should be used with caution in combination with prescription medications for heart conditions, as it may enhance their effects. Consulting a healthcare provider before use is recommended. Possible side effects include mild dizziness, nausea, or gastrointestinal discomfort in sensitive individuals. There isn't enough reliable information to know if hawthorn is safe when used for longer than 16 weeks. Hawthorn is usually well-tolerated.
References:
Dahmer S, Scott E. Health effects of hawthorn. Am Fam Physician. 2010;81(4):465-468.
https://www.webmd.com/vitamins/ai/ingredientmono-527/hawthorn

TAXONOMY
kingdom: Plantae
order: Rosales family: Rosaceae genus: Crataegus
species: Crataegus oxyacantha
COMMON NAME
Hawthorn, May Tre, Quickthorn, White Thorn, Aubépine (French)
FLOWERING TIME IV–VI month