

BSBI CONTACTS
President PAUL ASHTON
Chair of the Trustees
Hon. General Secretary
SANDY KNAPP
Dept of Biology, Edge Hill University, St Helens Road, Ormskirk, L39 4QP president@bsbi.org +44 (0) 1695 650931
Natural History Museum, Cromwell Road, London, SW7 5BD chair@bsbi.org +44 (0) 942 5171
BARRY O’KANE hongensec@bsbi.org
Company Secretary and registered address MKS LLP Chartered Accountants 4 Beaconsfield Road, St Albans, AL1 3RD +44 (0) 1727 838255
Chief Executive JULIA HANMER 65 Sotheby Road, London, N5 2UP julia.hanmer@bsbi.org +44 (0) 7757 244651
MEMBERSHIP SUPPORT AND ADMINISTRATION
Hon. Membership Secretary (subscriptions and changes of address) and BSBI News distribution
Finance Manager (all financial matters except Membership)
Communications Officer (including publicity, outreach and website; British & Irish Botany)
Fundraising and Engagement Manager
Administration Officer (general enquiries about BSBI’s work)
Hon. Field Meetings Secretary (including enquiries about field meetings)
Panel of Referees and Specialists (comments and changes of address)
SCIENCE AND DATA
Head of Science
Scientific Officer (& Panel of VCRs –comments and changes of address)
Database Officer
Data Support Officer
COUNTRIES AND TRAINING
Countries Manager
Training Coordinator (FISC and Identiplant)
England Officer
GWYNN ELLIS 41 Marlborough Road, Roath, Cardiff, CF23 5BU gwynn.ellis@bsbi.org +44 (0) 2920 332338
Please quote membership no. on all correspondence.
JULIE ETHERINGTON Church Folde, 2 New Street, Mawdesley, Ormskirk, L40 2QP
julie.etherington@bsbi.org +44 (0) 7944 990399
LOUISE MARSH louise.marsh@bsbi.org +44 (0) 7725 862957
SARAH WOODS 23 Bank Parade, Otley, LS21 3DY sarah.woods@bsbi.org +44 (0) 7570 254619
JENNA POOLE jenna.poole@bsbi.org +44 (0) 7404 231178
JONATHAN SHANKLIN 11 City Road, Cambridge, CB1 1DP fieldmeetings@bsbi.org +44 (0) 1223 571250
JO PARMENTER jo.parmenter@tlp.uk.com +44 (0) 7710 252468
KEVIN WALKER kevin.walker@bsbi.org +44 (0) 7807 526856
PETE STROH peter.stroh@bsbi.org +44 (0) 7981 572678
TOM HUMPHREY tom.humphrey@bsbi.org
JAMES DREVER james.drever@bsbi.org
JAMES HARDINGMORRIS james.harding-morris@bsbi.org +44 (0) 7526 624228
CHANTAL HELM chantal.helm@bsbi.org +44 (0) 7896 310075
SAM THOMAS sam.thomas@bsbi.org
Wales Officer (Priority Plants Project) ALASTAIR HOTCHKISS alastair.hotchkiss@bsbi.org
Scotland Officer
Ireland Officer
MATT HARDING matt.harding@bsbi.org +44 (0) 7814 727231
BRIDGET KEEHAN bridget.keehan@bsbi.org +353 (0) 86 3456228
Training Officers (Northern Ireland) JO MULHOLLAND
PUBLICATIONS
BSBI News Editor
British & Irish Botany Editor-inChief
Book sales agent
KIM LAKE jo.mulholland@bsbi.org +44 (0) 7936 303429 kim.lake@bsbi.org +44 (0) 7448 345167
JOHN NORTON bsbinews@bsbi.org +44 (0) 2392 520828
STUART DESJARDINS bib@bsbi.org +44 (0) 7725 862957
PAUL O’HARA Summerfield Books, Penrith, CA11 0DJ info@summerfieldbooks.com +44 (0) 1768 210793
BSBI website: bsbi.org BSBI News: bsbi.org/bsbi-news Tel: +44 (0) 7404 231178
A WORD FROM THE PRESIDENT 1
ARTICLES
More common problems in identification
Bob Leaney 3
Seeing red (or is it blue?) Simon Harrap 10
Trollius europaeus (Globeflower) in the West Pennine Moors and experience in propagation for re-establishment Peter Jepson 13
Mrs Patty Charlotte O’Brien (Miles) 1817–1891
The Lady of the Flowers David C. Rayment 17
The extinct Glenridding Hawkweed Hieracium subintegrifolium discovered in two new sites at Ingleborough, Yorkshire Brian Burrow 20
BEGINNER’S CORNER
Speedwells (Veronica) Part 3 Mike Crewe 22
ADVENTIVES & ALIENS
Adventives & Aliens News 36
Compiled by Matthew Berry 25
Alien plants in the greater Dublin area in 2024
Sylvia Reynolds 39
Sorbus incana (Silver Whitebeam) on Brownsea Island (v.c. 9), new to Britain and Ireland David Leadbetter 47
An overlooked non-native variant of Primula veris (Cowslip) originating from wildflower seeds and plantings David Broughton 49
SPECIAL ARTICLE
A new BSBI website designed for our botanical community Sarah Woods 53
Contributions for future issues should be sent
NOTICES
News, events and updates on the work of the BSBI and its members, including: dates of the forthcoming AGM, British and Irish Botanical Conference and New Year Plant Hunt; welcome to new staff; call to join the Skills and Training Committee; update on FISCs in Ireland; Wild Roses of Great Britain and Ireland new handbook; Panel of VCRs; contents of British & Irish Botany 7:2. 56

Cover photo: Allium scorodoprasum (Sand Leek), Clun Castle, Shropshire (v.c. 40). John Martin (see Country Roundups, p. 62)
The Botanical Society of Britain and Ireland (BSBI) is the leading charity promoting the enjoyment, study and conservation of wild plants in Britain and Ireland. BSBI is a company limited by guarantee registered in England and Wales (8553976) and a charity registered in England and Wales (1152954) and in Scotland (SC038675). Registered office: 4 Beaconsfield Road, St Albans, AL1 3RD. All text and illustrations in BSBI News are copyright and no reproduction in any form may be made without written permission from the Editor. The views expressed by contributors to BSBI News are not necessarily those of the Editor, the Trustees of the BSBI or its committees. BSBI ©2025 ISSN 0309-930X
A WORD FROM THE PRESIDENT
Recording is one of the key areas of work for the BSBI and its members. With the new BSBI Recording App, making such records is now easier than ever. Simply go to the new BSBI Documentation website: docs.bsbi.org to find out more. By the time you read this autumn will be well on its way but that doesn’t mean that recording can cease. Trevor Dines’ excellent new book Urban Botany (see review p. 79) shows that urban plants have a longer flowering season, so explore your local urban area and use the app. The records that you make all flow into the BSBI Distribution Database, to which you have access as a member. It is a fantastic resource covering all of Britain and Ireland and going back over many decades. This means it can be used to understand plant distributions at a geographic and temporal level. I think it is probably the most comprehensive set of national plant records and one in which members can be justifiably proud.
The Database also underpins the second of the BSBI’s key areas, that of publications of books and papers. The new GB Red List for vascular plants by Pete Stroh et al. is due to be published online this autumn as a special issue of British & Irish Botany
EDITORIAL
This issue includes the final part of Mike Crewe’s look at Speedwells (Veronica spp.) for Beginner’s Corner. We would welcome suggestions for topics to cover in future issues – either regarding identification of groups of commoner species – or on other aspects of field botany that haven’t been covered before or could benefit from an update. The series started in 2018 with a look at hand lenses, followed by articles on bud burst phenology of trees and shrubs, ticks, herbaria, ferns and recording grid references with smartphones and GPS. I would always be happy to receive articles from other contributors.
For beginners and ‘improvers’ I am pleased to present another in Bob Leaney’s series of guides to
This details our most threatened plants and is a direct product of the BSBI Database. This autumn also sees the publication of a new BSBI Handbook on Roses (see back cover of this issue and notice on p. 59). This is the second handbook the Society has published this year, hot on the heels of Angus Hannah’s fine Brambles of Scotland. It’s very rare to have two handbooks published in one year. Having been involved as editor with the BSBI Handbooks in the past I know how much effort is needed by the authors to see such a work through to publication, so my congratulations to all involved.
You will be able to view the books and meet other members face-to-face at this years British & Irish Botanical Conference on 29 November at Edge Hill University, Lancashire (see notice on p. 56). This is my home patch and I look forward to meeting many of you there. If books and meeting me aren’t enough to tempt you then let me add that there will be a fine collection of talks and activities on various botanical subjects. How can you resist?
Paul Ashton president@bsbi.org
‘common problems in identification’ – the previous on Mints was back in issue 153 (April 2023). This instalment (p. 3) solves four different problems, three of which relate to vegetative identification of basal leaves and younger leaves of similar-looking species that develop over winter or in early spring. The article should therefore help with botanising over the coming season. More from Bob will be included in the winter issue, due out by late January.
John Norton john.norton@bsbi.org

More common problems in identification
BOB LEANEY
Here are presented some more ‘common problems’ encountered during recording sessions of the Norfolk Flora Group. This time, because the group has for several years been recording in the winter, many of the topics involve vegetative identification of basal leaves found in the winter and early spring.
As always the material presented is the result of notes, photocopies and drawings made on numerous specimens I have taken home after recording sessions, specimens where the group has found it difficult to reach a reliable identification in the field. The diagnostic characters and measurements discussed come from the usual standard texts listed in the reference section but there are also some novel characters and measurements made by myself or others in the group.
Medicago sativa vs Melilotus spp. (vegetative identification)
Early in the year, before flowering, Medicago sativa subsp. sativa (Lucerne) and species of Melilotus (Melilots) can look very similar, erect in habit and
Basal leaf rosettes (and flowers) of Erophila verna (Common Whitlowgrass). Mike Crewe
with very similarly shaped obovate leaflets. They frequently cause problems with identification, but can in fact be quite readily separated on stipule shape and the distribution of the serrations on the leaflets. Like all Medicago spp. Lucerne has broad-based, clasping stipules and leaflet serrations confined to the distal third or so of the leaflet; in Melilotus spp. the stipules are fine and awl-shaped, without a broad clasping base, and the leaflet serrations extend down onto the lower half of the leaflet (i.e. only the lower third or so of the leaflet is entire edged).
In Medicago subsp. falcata (Sickle Medick) and M. sativa nothosubsp. varia (Sand Lucerne) the stipules will be similar to those in subsp. sativa, but the leaflets are more narrowly obovate and both plants are much less robust and more trailing in habit, rather than erect. It is not possible to separate M. sativa subsp. falcata and nothosubsp. varia vegetatively, and the same goes for the Melilot species.
More common problems in identification
MEDICAGO SATIVA
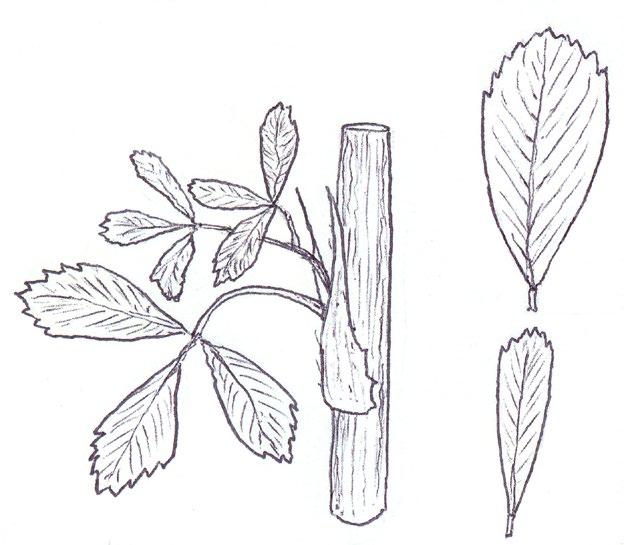
Subsp. sativa
Broad-based clasping stipules
Serrations confined to distal third of leaflet
c. 1/3
Subsp. falcata and nothosubsp. varia
Tiny overwintering and spring basal rosettes
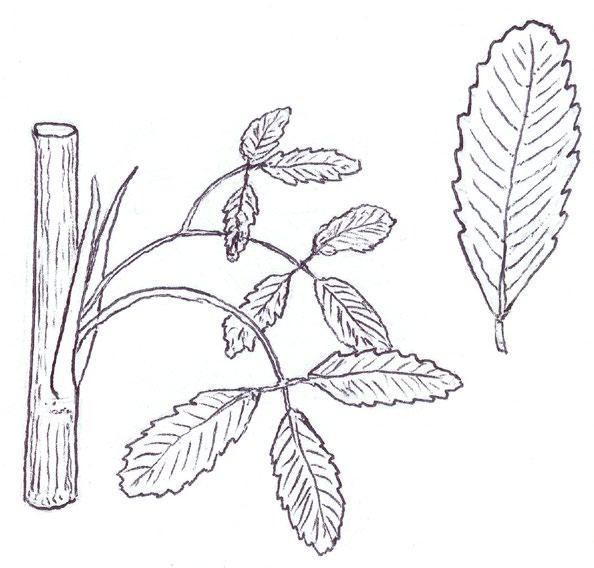
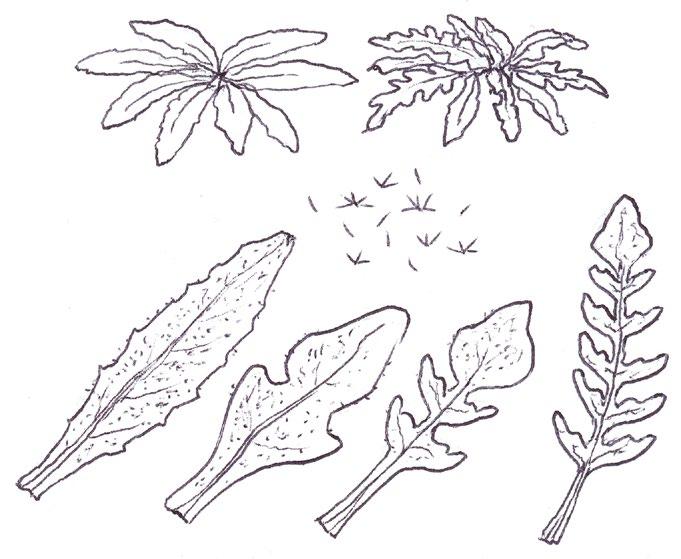
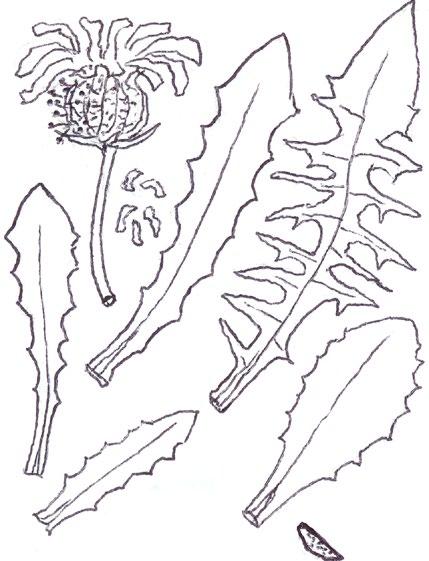
Tiny rosettes of the annual crucifers Erophila verna agg. (Common Whitlowgrass and related species), Arabidopsis thaliana (Thale Cress), Capsella bursapastoris (Shepherd’s-purse) and Sisymbrium officinale (Hedge Mustard) present frequent problems from early winter though until spring. The basal leaves of Erigeron canadensis (Canadian Fleabane) can also be tiny and one genotype can look surprisingly like Arabidopsis thaliana at this stage; one also has to look out for the succulent, more or less spathulate leaves of Saxifraga tridactylites (Rue-leaved Saxifrage), Montia fontana (Blinks) and Claytonia perfoliata (Springbeauty) in ruderal or pavement situations. All these plants are actually quite easily distinguished using the leaf shape, colour and indumentum characters illustrated here.
There seems to be be two very different common genotypes of Erigeron canadensis in our area, the typical one with very attenuated petioles and 1–2 (3) forwardly pointing triangular lobes near the leaf tip; and one, more resembling Arabidopsis, where the leaf is broadly ovate with a very short petiole and one tiny
MELILOTUS spp.
c. 2/3
Fine awl-shaped stipules without clasping base
Serrations on upper two thirds of leaflet
blunt lobe near the tip. Despite the very different leaf shape, both entities have the same regularly spaced out ciliate hairs on the petiole and typical forwardly curved hairs on the leaf edge further up. Basal leaf rosettes of Erigeron sumatrensis (Guernsey Fleabane) are usually much larger (5–10 cm across) and the leaves have 3–5 crenate-serrate lobes; the ciliate hairs on the petiole are sparser and interspersed with many shorter kinked hairs between. The basal leaves of E. floribundus (Bilbao Fleabane) are usually very much larger (10–15 (20) cm), shiny dark green and with numerous, very long, parallel-sided lobes.
Erophila verna agg. can only be reliably separated into the three species treatment used by Rich (1991) and Stace (2019) when in flower, so 8-figure grid references should be taken so that presumptive identifications of E. glabrescens (Glabrous Whitlowgrass) and E. majuscula (Hairy Whitlowgrass) can be checked later in the spring.
The Norfolk Flora Group has recently been finding populations of E. glabrescens, having noticed that the leaves of this species are noticeably pale yellowish-green and have very long petioles, about
EROPHILA VERNA GROUP
E. glabrescens
Pale yellowgreen
Petiole : blade ratio
c. ½ c. ¾
E. verna s.s. c. 2×
ARABIDOPSIS THALIANA
Typical Occasional
Dark shiny green
Basal leaves with truncate terminal lobe
Lobes with toothed lower edge
SISYMBRIUM OFFICINALE
More common problems in identification
CAPSELLA BURSA-PASTORIS
Very variable leaf dissection
Pale dull green
Lobes with smooth rounded lower edge
ERIGERON CANADENSIS & SUMATRENSIS
Broad leaved genotype
E. canadensis
Typical form
E. sumatrensis
Leaves simple at first, soon becoming trifid
SAXIFRAGA TRIDACTYLITES
All succulent, glabrous
No proper rosette Trailing decumbent habit Pale yellow green
MONTIA FONTANA
1(3) lobes
Early leaves narrowly spathulate
Later leaves simple rhombic Pale yellow green
CLAYTONIA PERFOLIATA
E. majuscula
twice as long as the blade; the leaves are usually completely glabrous except for the leaf edge but there are often just one or two hairs on the lamina upper surface near the leaf tip. In E. verna s.s. the petiole is about three-quarters the length of the blade and the leaves are mid green, with moderate hairiness; E. majuscula has very densely hairy leaves, a similar mid green colour and the petiole is less than or equal to half the length of the blade.
Arabidopsis thaliana basal leaves, like those of Erophila , have a mixture of simple, forked and 3-rayed stellate hairs and are dark green and pretty much constant in shape – usually broadly ovate to elliptic with 2–3 obscure teeth around half way up and a very short petiole; in Norfolk we rarely see narrowly ovate to elliptic basal leaves with long petioles as in the Rich (1991) illustration.
The basal leaves of Capsella bursa-pastoris are extraordinarily variable in any one population or even in one plant! Usually, however, at least some will have pinnatisect or partially pinnatisect leaves with characteristic forwardly curved lobes showing an entire lower edge. Simple leaves found on their own cause the most difficulty, but with experience can usually be identified by their numerous tiny teeth, shape, pale green colour and combination of simple and (3)5 rayed stellate hairs.
The basal leaves of Sisymbrium officinale are very constant and readily identifiable by their dark, shiny green toothed pinnate lobes and characteristic truncate terminal lobe (not found on the otherwise very similar stem leaves later on).
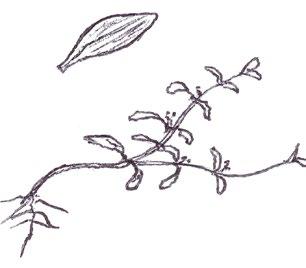
Saxifraga tridactylites, Montia fontana and Claytonia perfoliata have basal leaves that are all slightly succulent, glabrous and more or less spathulate. The saxifrage usually grows on walls, in road and mud collected at wall bases, or in our region, in the depressions between the flint cobbles of decorative paving. Its rosettes are very tiny, around 1 cm across, and the leaves are characteristically held in a high arched posture. Early on this may be all there is to go on, but later a few ‘give-away’ shallowly trifid leaves will be produced; eventually deeply trifid leaves will begin to show in the centre of the rosette. Montia fontana can be an aquatic but nearly all our plants
grow either in damp hollows in amenity grassland or on road kerbs or in gutters. Blinks has a trailing habitat and does not produce a leaf rosette; the leaves are spathulate to obovate throughout. Claytonia perfoliata can be spotted by its striking pale yellowgreen colour; the first leaves are very narrowly spathulate and long petioled, and it can be many weeks before the broadly rhombic leaves appear.
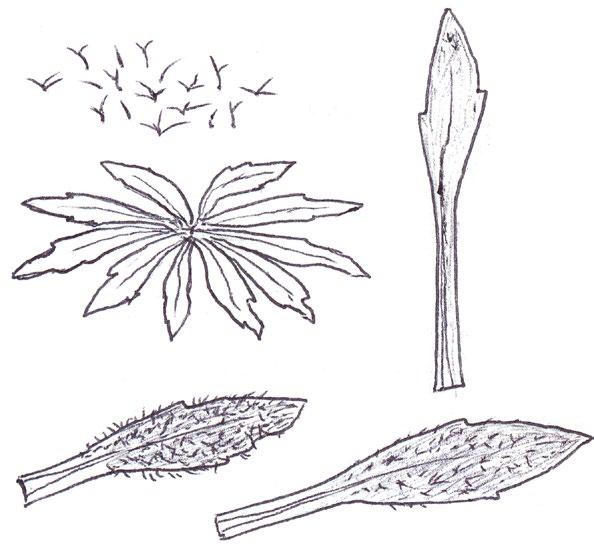
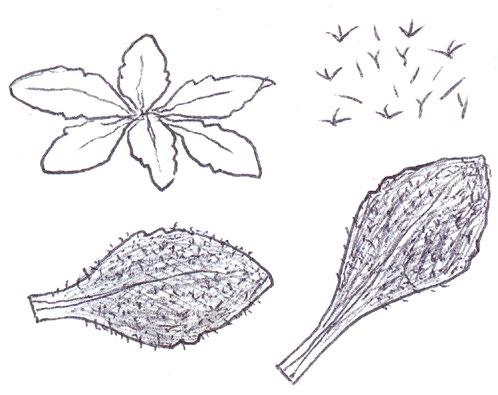
Overwintering and spring basal leaves of common yellow composites
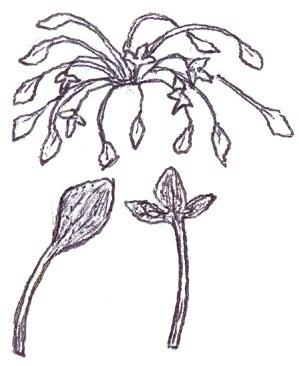
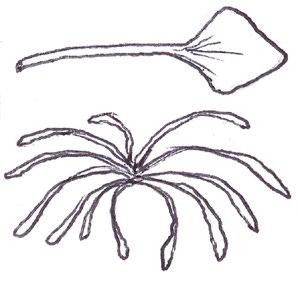
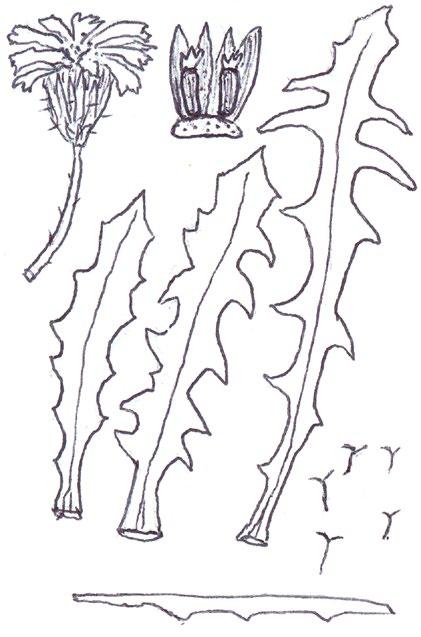
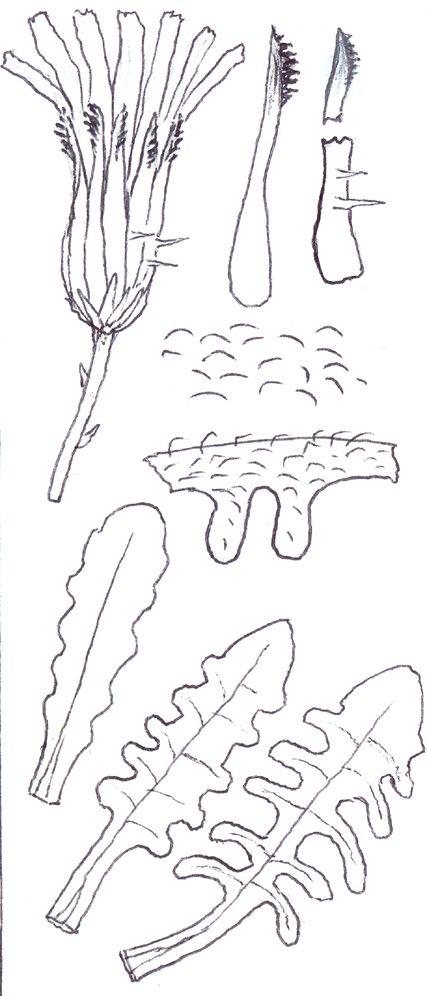
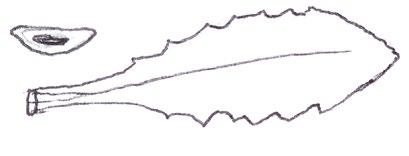
Since embarking on winter recording the basal leaves of Hypochaeris radicata (Cat’s-ear), Scorzoneroides autumnalis (Autumn Hawkbit), Crepis capillaris (Smooth Hawk’s-beard), C. vesicaria (Beaked Hawk’s-beard), Leontodon saxatilis (Lesser Hawkbit) and less often L. hispidus (Rough Hawkbit) have been causing the group problems in almost every recording session. The basal leaves of these species are extraordinarily variable and frequently produce ‘lookalikes’ mimicking other species in the group – a few of these variations are illustrated in the BSBI Plant Crib (Rich & Jermy, 1988) and Sell & Murrell vol. 4 (Sell & Murrell, 2006) and more are shown here. It is important to realise that the simple obovate leaves of Crepis capillaris can be very like immature or shade leaves of Taraxacum (dandelions) and may not be separable vegetatively; also that the tiny winter leaves of this species can also closely resemble Hypochaeris glabra (Smooth Cat’s-ear).
Vegetative separation of these taxa on basal leaves is not always possible, but usually a reliable identification can be arrived at using:
• the shape of the leaf lobes (rounded, long and parallel-sided, triangular);
• the width of the terminal leaf segment compared with the width of the undissected leaf below;
• texture (thin and membranous, thick and leathery) and indumentum.
It is not only Leontodon hawkbits that have characteristic hairs; the hairs of the other species are not illustrated in the literature and difficult to describe, so hopefully the drawings here will be helpful.
LEONTODON SAXATILIS
HYPOCHAERIS RADICATA
LEONTODON HISPIDUS SCORZONEROIDES AUTUMNALIS
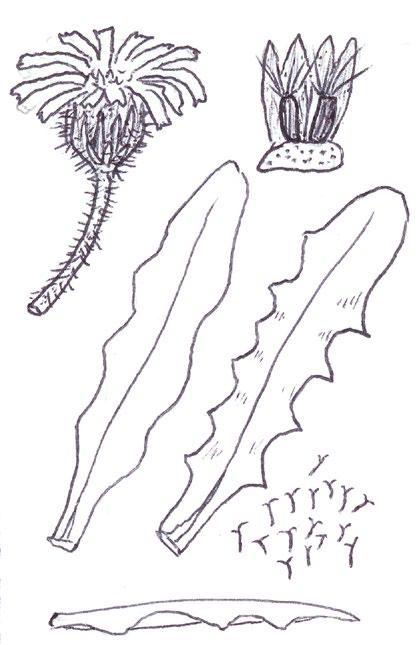
CREPIS CAPILLARIS

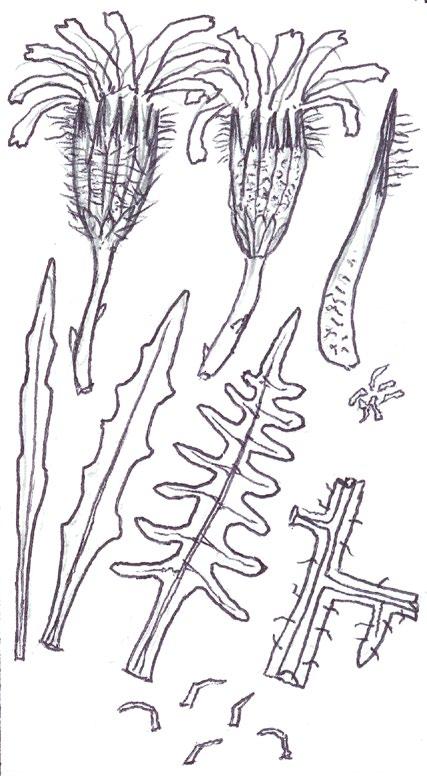
CREPIS VESICARIA
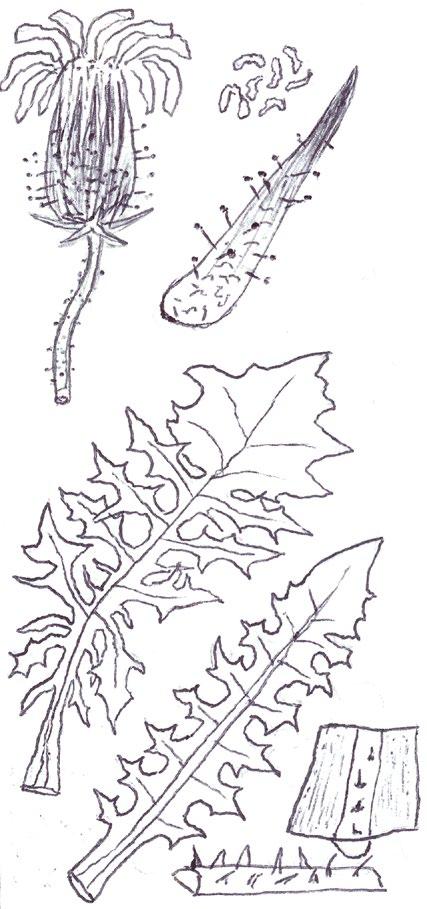
TARAXACUM
HYPOCHAERIS GLABRA
Leontodon saxatilis
Sparse forked hairs; fairly thin textured leaves; typically with sinuate leaf edge but on occasions can have very long triangular lobes which may be hooked forward or backward; leaf edge flat (not undulate). Leaf rosettes in favoured lawn type grass short leaved and fairly appressed to the ground, but in longer grass the leaves can be very long and ascending.
Leontodon hispidus
Dense forked hairs; thick leaf texture; leaf edge always sinuate to shallowly sinuate dentate; leaf edge usually undulate.
Scorzoneroides autumnalis
Usually has sparse once or twice kinked hairs (only occasionally glabrous); fairly thick textured leaves; leaf lobes usually long and parallel sided with obtuse (not broadly rounded) tips; very long lobed forms can have hardly any leaf lamina and long narrow ‘lobes on its lobes’; occasionally produces extraordinary long, linear, more or less entire leaves; terminal segment same width or narrower than width of the undissected part of the leaf below.
Hypochaeris radicata
Leaves seldom glabrous and nearly always with quite dense, arched hairs; thick textured; fairly wide terminal segment; leaf lobes usually short but can be very long and very occasionally have lobes on the lobes; leaf lobes always have broadly rounded tips.
Crepis capillaris
Usually glabrous, sometimes with a few weak bristles on undersurface midribs; very thin textured; winter leaves very small (only a few centimetres in length) and very constant with shallowly sinuate dentate edge; spring leaves are typically sinuate dentate, with terminal segment broader than the leaf below, but large leaved forms can have extremely long triangular lobes, with on occasions large triangular teeth on the lobes very like Crepis vesicaria (separation only possible if the latter has the diagnostic hairs described below).
Crepis vesicaria
The winter leaves are very long, dark green and strap shaped with fairly long, toothed triangular lobes, forming very characteristic dense, many leaved and appressed rosettes. The spring leaves have much longer lobes with long curved teeth, similar to those that Crepis capillaris can produce and already described. These types of leaves can only be ascribed to Crepis vesicaria if the diagnostic indumentum is present: either short, stout red bristles on the upper surface midribs (Poland & Clement, 2020) or longer, thinner hairs on the under surface midribs and lamina; all these hair types can be absent.
Glabrous leaves like this should not be identified, as they can be mimicked by both Crepis capillaris and Taraxacum , flowering plants of which can produce similar basal leaves to the spring leaves here illustrated.
Taraxacum
It is important to realise that immature late winter leaves or shade leaves of some Taraxacum microspecies can mimic the simple oblanceolate to obovate leaves of Crepis capillaris – both are usually glabrous or have just an occasional weak bristle on the undersurface midrib. Separation may be possible by looking for the hollow petiole of Taraxacum, but this can be difficult in small leaves; also note Crepis species can produce some white latex on squeezing the petiole, just like Taraxacum
Hypochaeris glabra
The very small basal leaves of this species very much resemble the tiny, single winter leaves of Crepis capillaris. They are always very small and glabrous except for the presence of fine ciliate hairs on the leaf edge, which are diagnostic. However, I’m not sure if ciliate leaf edge hairs are always present. Tiny glabrous leaves in spring or early summer on very poor soils should be gridded for confirmation when in flower if the record is thought to be a significant one.
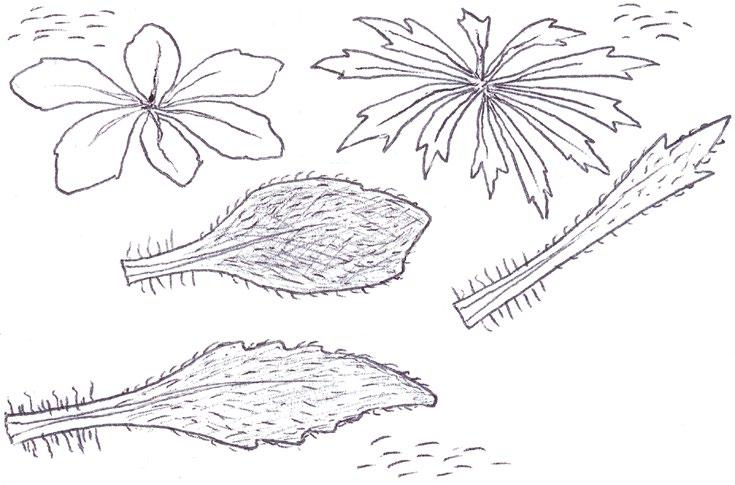
Identification of Anthemis and Cota
In Norfolk Anthemis cotula (Stinking Chamomile) is usually found on the heavy boulder clay south of Norwich and A. arvensis (Corn Chamomile) on the light sandy or chalky soils of the west. Over the last decade or so, however, we have been finding A. arvensis outside its native range and away from its usual arable habitats, in villages or suburbs, often as part of an obvious seed mixture sowing of ‘wildflowers’. We have also become gradually aware that Cota austriaca (Austrian Chamomile) is now to be found more commonly in such situations, largely since we realised that, compared with the Anthemis species, this plant is much more robust and thick stemmed, extremely hairy and much more strongly aromatic. Contrary to the usual descriptions, the two Anthemis spp. are both mildly scented and A. cotula does not smell particularly unpleasant; furthermore, the standard characters used to separate the two, hairiness and the shape of the terminal leaf segments, are far from clear cut and do not correlate with the shape of the receptacle scales.
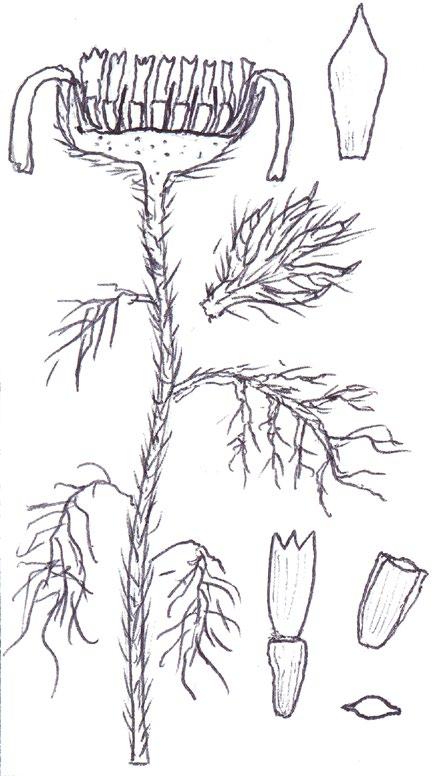
More common problems in identification
In separating the species of these two general from each other, and from the mayweeds Tripleurospermum inodorum (Scentless Mayweed) and Matricaria chamomilla (Scented Mayweed), one has to become adept at thumbnail dissection of the flowerhead, so as to obtain a more or less central, vertical transection of the capitulum. T. inodorum is usually spotted because of its long, fresh green and finely divided leaves and long ligules; in M. chamomilla the leaves are not so long and the short ligules are nearly always strikingly reflexed. Confirmation is by finding a solid receptacle in T. inodorum and a vertically elliptical cavity in the receptacle of M. chamomilla. No receptacle scales should be found – these are characteristic of both Anthemis and Cota. The best way to find receptacle scales in the field and assess their shape is not to look for them in a thumbnail vertical transection, where they are very easy to miss between the disc florets, but to pinch off all the disc florets and rub them between finger and thumb into the palm of the hand for lens examination. On the other hand,
COTULA
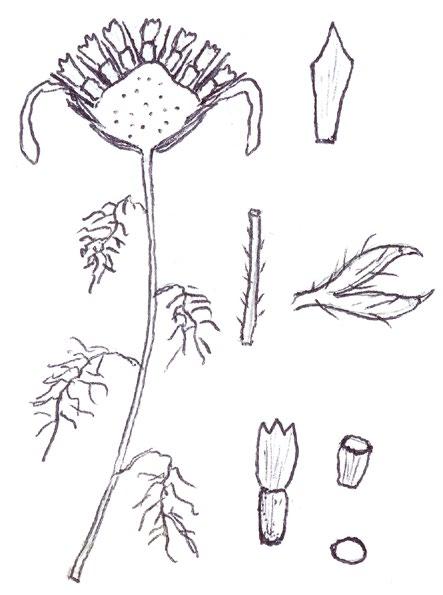
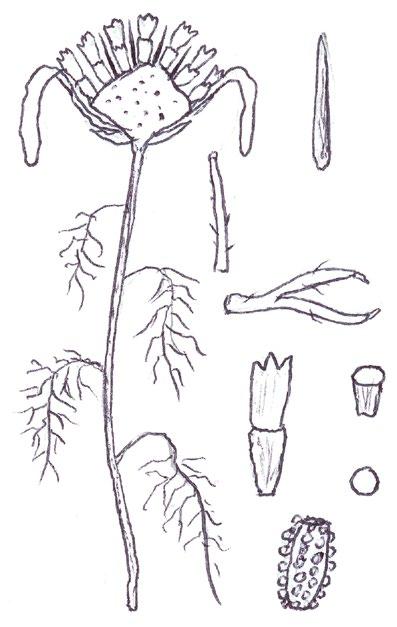
COTA AUSTRIACA
COTA TINCTORIA (differences): greyish white colour, yellow ligules
ANTHEMIS ARVENSIS ANTHEMIS
their position on the capitulum and the shape of the capitulum are best looked for on a transection at home with a microscope.
I have taken home numerous Anthemis and Cota specimens for microscopy of a vertical transection and reached a different identification to that suspected in the field on many occasions: either because I have been able to find the linear receptacle scales of A. cotula confined to the central/upper part of the capitulum, or because the vertical transection revealed the more or less flat capitulum of Cota austriaca – in both Anthemis species the capitulum is strikingly conical.
Anthemis tinctoria (Yellow Chamomile), which used to be a fairly frequent find in urban habitats, has recently almost disappeared from the county, so I have not been able to confirm if this taxon also has a flat or near flat capitulum. It is certainly a very robust and very strongly scented plant like Cota austriaca, but with whiter leaves and of course, yellow ligules. Both Cota species have compressed
Seeing red (or is it blue?)
SIMON HARRAP
WhenI first started to photograph plants seriously in the 1990s I used slide film. Fairly quickly I came across a very significant limitation: plants with unquestionably blue flowers, if photographed in sunlight, would reproduce as reddish-purple on the slide. The very same plant, in the same place, if photographed under overcast conditions or in shadow, would reproduce as a more or less true-to-life blue. Hyacinthoides non-scripta (Bluebell) is one of the classic examples; if you search out published photographs of Bluebells in older books or even old postcards you will find examples of ‘pinkbells’. One of the most extreme cases of this blue-to-red transformation was Pinguicula grandiflora (Large-flowered Butterwort) which I photographed
and more or less keeled (not cylindrical) achenes, and this character can already be seen in the translucent, pale green, immature achenes found at anthesis, when these plants are usually spotted. It should be noted, however, that the tuberculate ribs on the achenes of A. cotula are not present at this stage.
References
Poland, J. & Clement, E.J. 2020. The Vegetative Key to the British Flora (2nd edn). John Poland, Southampton.
Rich, T.C.G. 1991. Crucifers of Great Britain and Ireland. BSBI Handbook No. 6. Botanical Society of the British Isles, London.
Rich, T.C.G. & Jermy, C. 1998. Plant Crib 1988. Botanical Society of the British Isles, London.
Sell, P.D. & Murrell, G. 2006. Flora of Great Britain and Ireland, Volume 4: Campanulaceae–Asteraceae. Cambridge University Press, Cambridge.
Stace, C.A. 2019. New Flora of the British Isles (4th edn). C & M Floristics, Middlewood Green, Suffolk.
Bob Leaney
122 Norwich Road, Wroxham, NR12 8SA
in the Burren: in sunshine pink, in shadow blue (Figure 1).
I have no idea what the basis in physics is for the effect – perhaps in sunlight the plant reflects a lot of infrared which we cannot see but which is picked up by the emulsion on the slide film and then transposed into the visible red? Whatever the cause, it seemed strange to me that the authors, photographers and publishers of books featuring ‘pinkbells’ did not pick up on the error.
Authors (myself included) often work from photographs and/or herbarium specimens. Herbarium specimens seldom retain the true colours of the living plant, but this is usually obvious and therefore errors are mostly easily avoided. If the
colours on a photograph are misleading, however, one can see how errors can creep into the written word. It can be easier to visualise a photograph with false colours than it is to remember the real thing last seen months, years or even decades earlier. It is not just in the process of capturing an image that errors can appear; reproduction is fraught with problems. When we were working on Orchids of Britain & Ireland: A Field and Site Guide (Harrap & Harrap, 2006) we came across a whole range of frustrating issues. Most of the photographs reproduced in the book were taken on slide film, and for reproduction these were professionally scanned to produce a digital image. Try as we might, however, we could not get the colours right – rich pinks and magentas as in the genera Gymnadenia, Dactylorhiza, Orchis and Anacamptis were dulled. If you are lucky enough to have a copy of the book, take a look at the photos, or better still take it out into the field and compare it with the real thing. It was (and is) heartbreaking. It was not our fault, however, or the fault of the publishers or printers.

and
Seeing red (or is it blue?)
It is just a fact of life. Offset lithography (sometimes just ‘offset’ or ‘litho’), the technique still used for most commercial printing, almost always uses just four inks: cyan, magenta, yellow and black (CMYK for short). These four inks are combined to produce a vast range of colours, but there are some colours that they cannot produce – these colours are ‘out of gamut’. Unfortunately for us, our orchids’ magentas and pinks were well out of gamut and could not be printed by standard 4-colour litho, hence the muddy results. It is possible to expand the gamut of colours that can be reproduced, but that involves adding extra inks (see below) and is expensive – it is usually reserved for high-end fine art books, etc.
Digital imaging has transformed photography. A digital camera’s sensor collects data for three colour ‘channels’, red, green and blue (RGB for short) and these three colours are later mixed by computer to produce millions of colours. Digital images work especially well when viewed on screen, because screens use the same three colours (RGB) to recreate the image. All good so far, but RGB processes also

The slides were photographed on a light box and the raw digital images processed identically. On the slide taken in sunshine the flowers are reddish-purple; taken in the shade the same flowers are blue. But, in order to be reproduced here in print the digital images have to be converted from RGB to CMYK, which changes the colours again (they are duller and more muted).
Figure 1. Pinguicula grandiflora (Large-flowered Butterwort), Holywell, Cappanawalla, The Burren, Co Clare, 22 May 2004. Photographs taken in shade
in sunshine on Fujichrome slide film (probably Fuji Sensia).
have colours that are ‘out of gamut’, and these are not the same as the CMYK ‘out of gamut’ colours! Helpfully, however, they seem to impact less on the reproduction of images of plants. The net result is that digital photos viewed on screen tend to be more accurate than the printed versions (try comparing photos on a tablet or phone with the same species in a book, and with the real thing out in the open air).
There are still issues with digital photographs, however. For commercial printing, the photograph that came off the camera as an RGB file has to be converted into CMYK, because the printing press still uses those four inks and still has the same limited gamut of colours that can be reproduced. I think that bright blue, violet and magenta flowers are the worst offenders: they simply cannot be printed accurately.
One recent case is the BSBI’s very own Violas of Britain and Ireland (Porter & Foley, 2017). Violets are, by their nature, prone to problems in reproduction: take a look at the photographs of Viola riviniana and V. reichenbachiana: the colours bear little relation to reality, but I cannot be smug, as the colours as printed of Common and Early Dog-violets in Harrap’s Wild Flowers are not much better!
What to do? It is possible to print more accurately, and some desktop printers use eight or even more inks to produce an expanded gamut, but this is not yet the answer for a large run of affordable books or periodicals. Publishing online and viewing on screen can help a lot, as noted above, but online field guides and periodicals have other limitations, and my understanding is that ‘e-book’ versions of many field guides, etc. take the CMYK files used for the printed version and simply convert them back into RGB, this conversion producing another loss of fidelity. I think that the best thing at present is to be aware
of the issues and not to attach too much weight to the exact appearance of blue, violet and magenta flowers in your field guide or BSBI handbook. It would also be helpful for authors, especially of more technical reference works, to explicitly point out the shortcomings of a reproduced image (and in this I will try to take my own advice!).
Finally, the issues surrounding sun vs shade and RGB vs CMYK are not the only problems encountered in recording and reproducing colours. Bright, very saturated colours, such as red poppies or yellow daffodils, also pose a challenge, and it is all too easy to ‘blow’ a colour and end up with a solid block of red or yellow with no texture or detail. Another issue is the algorithms used by many cameras to construct an image from the raw data coming from the sensor (after all, just a string of numbers), especially when on ‘auto’. The software writers know that people like vibrant, bright images, and so the finished picture on you digital camera or smartphone tends to be pushed in that direction. In short, digital photography offers the opportunity to produce fantastic true-to-life images, but care has to be taken at every step if you are to really take advantage of that opportunity.
References
Harrap, A. & Harrap, S. 2006. Orchids of Britain & Ireland: A Field and Site Guide. Bloomsbury Publishing, London & New York.
Porter, M. & Foley, M. 2017. Violas of Britain and Ireland. BSBI Handbook No. 17. Botanical Society of Britain and Ireland, Bristol.
Simon Harrap erigeron@norfolknature.co.uk

Trollius europaeus (Globeflower) in the West Pennine Moors and experience in propagation for re‑establishment
PETER JEPSON
In 2023 Natural England (Cheshire to England Team) commissioned me to undertake a study into previously known populations of Trollius europeaus (Globeflower) in and peripheral to the West Pennine Moors SSSI, in South Lancashire (v.c. 59). It was known that certain local sites had been lost in the last few decades but there was no up to date knowledge of all the sites. T. europaeus is considered of ‘least concern’ in Stroh (2014); whilst in Stroh (2023), it is considered relatively widespread in its core northern and western areas, but has clearly declined at the fringes of its range, a process which began before 1930 and still continues. A data search revealed sixteen sites in the wider area with nine sites within the SSSI boundary, six of which were known to me in the mid-1970s, with the seventh site for the last twenty five years.
Survey of previously known sites
Of the nine sites, T. europaeus only remained extant at two. The reason for the losses was attributed
Trollius europaeus (Globeflower) colony at Belmont, West Pennine Moors SSSI (v.c. 59), May 2023. Peter Jepson (photographs by the author)
to land management changes, either through the loss of seasonal grazing, increased tree cover or a combination of both. Most of the extinct populations were on valley side locations previously carrying open woodland, which were aftermath grazed in late summer in combination with their adjacent meadows following traditional haymaking. In this respect they constituted a form of wood pasture in a management regime that would comfortably fit with notions of ‘re-wilding’.
In terms of the two extant sites, one (Gale Clough) was small with only 34 blooms; its survival may be attributed to the fortuitous scrub clearance below overhead cables. The other (Belmont) was a large population growing on a field bank with over 1550 blooms in 2023 (photo above), a decrease of 48% from a count in 2000. This site had been receiving
Trollius
intermittent management for two decades, largely dependent on sheep sporadically wandering through a seasonally open gate and grazing the vegetation. At least some decline was associated with competition from Filipendula ulmaria (Meadowsweet) and the sward slowly becoming taller. The population had dipped before some cutting of the F. ulmaria after the T. europaeus has seeded resulted in a partial recovery of the latter.
Seed propagation for recovery
A part of the Natural England contract was to collect seed from populations and propagate at least 200 plants for a local recovery project. In order to ensure genetic diversity it was recognised that a proportion of seed should ideally be collected from outside the West Pennine Moors. The intention was from a site in the Forest of Bowland; however, most of the fruiting heads were missing, presumed due to slug predation, hence limiting the amount of seed available to harvest. Therefore, seed was collected from an additional site in the Yorkshire Dales.
Ripe fruiting capitula were collected during July 2023 and the seed allowed to fall (Figure 1). From each site 2.5 ml of the seed was sown separately in seed trays within days of collection (natural sowing). There appears to be a consensus on the internet that chilling the seed of T. europaeus promotes germination. The Seedaholic website recommends, if seeds fail to germinate after 6 weeks, to chill the seed tray in a refrigerator at 4°C for 8 weeks. The notion of placing such a tray in our domestic refrigerator did not gain favour; instead 2.5 ml of seed from two of the sites were each mixed with moist sand and stored in small containers in a refrigerator for 8 weeks at c.4°C before sowing (stratified sowing). The remaining collected seed was amalgamated and stored in cool dry conditions over the winter, one batch being sown in mid-February 2024 (late winter sowing) and another in mid-March 2024 (early spring sowing).
The natural and stratified trays were kept in a polytunnel until the end of October and then put outside until the first few days in February, whereupon they were returned to the polytunnel.

The late-winter and early-spring 2024 sowings were kept outdoors for c.8 weeks before being brought into the polytunnel.
Two seedlings appeared from the natural sowing during the autumn but died off during late winter. By the end of February there was abundant germination in all trays (Figure 2), although the germination arising from the stratified sowing was marginally later. No seedlings appeared from either the late winter or early spring sowing in the following 12 months.
Overall the germination was good, resulting in many hundreds of seedlings. During 2024 a proportion was potted up for the local Globeflower recovery project. As a pilot a few of these were planted out in a local species-rich meadow in early spring 2025, however, these suffered in the drought that followed. Most of the potted up seedlings that germinated in 2023 flowered well in 2025.
A contribution to the recovery project was made in spring 2023 by the daughter of two of my deceased friends, by way of the donation of a large T. europeaus plant from her parent’s garden. The plant (Roy’s Plant) was of known provenance having been grown from seed collected locally several decades previously. At the time the plant was already in leaf and with numerous flower buds. The plant was split into a good number of individual crowns with all
Figure 1. Seed of Trollius eurpaeus collected in July 2023.


above ground growth removed prior to being potted individually. These were grown on in a polytunnel and by September 2023 several of the plants were sufficiently established to give a late flowering.
Potential limitation to sustainable recovery
In January 2024 my attention was drawn to the BSBI species account for T. europaeus (Stroh, 2015) and the potential adverse implications in particular paragraphs for delivering sustainable species reestablishment. In citing Pellmyr (1989) and Hemborg & Després (1999) the account states that, Trollius europaeus is an obligate outcrosser and depends almost exclusively on a seed parasite, small anthomyiid (Chiastocheta) flies, for pollination. Also in considering threats, Lemke & Porembski (2013) are cited in terms of dependence on Chiastocheta flies and that a decline in the local population of this pollinator is likely to impact upon small and declining populations of T. europaeus leading to a limitation of pollen quantity and a higher likelihood of selfing or inbreeding. However, the latter statement of selfing is at odds with the earlier statement that Trollius europaeus is an obligate outcrosser.
In these respects it would be reasonable to assume that a Trollius europaeus recovery project would need to comprise not just the plants but it also Chiastocheta flies to be successful in the long term. Suchan et al. (2015) hypothesize that if this interaction between flower and fly is specific and obligate, then T. europaeus should experience dramatic drop in its relative
fitness in the absence of Chiastocheta. However, in their research this hypothesis was not proven, as T. europaeus populations without flies demonstrate a similar relative fitness to those with the flies present, contradicting the putative obligatory nature of this pollination system. They also observed that many other insects also visit and carry pollen among T. europaeus flowers. Indeed this later point agrees with my observations that other insects regularly visit the flowers of T. europaeus, even bumblebees.
Investigations in July 2024 by experienced entomologists studying Chiastocheta failed to detect any of these flies at the Belmont site. Similarly none were found at the Bowland site either, although they are known elsewhere in the vicinity. They have also been recorded in the nearby Yorkshire Dales.
The Belmont, Bowland and Yorkshire Dales populations each produced abundant well-formed seed, which germinated in quantity in the following spring (Figure 1).
Further, the quote in Stroh (2015) that T. europaeus is an obligate outcrosser needs further assessment. Growing in the polytunnel, several of Roy’s Plants came into bloom during September 2023, however, it was not realised until mid-winter that two fully formed seed heads on different plants had developed. On examination it was found that both had dehisced but only one still retained a few seeds. Nine seeds were extracted and examined and found to be well formed. These were sown in January 2024 and by mid-summer one seedling had appeared. As both of the plants involved had been split from a single large
Figure 2. Young seedlings of Trollius europeaus growing in a seed tray, March 2024.
Trollius europaeus (Globeflower) in the West Pennine Moors and experience in propagation
clump, they must be genetically identical. Whether they were pollinated by insects or self-pollinated is immaterial as it amounts to the same. However, given their isolation and the time of the year it is reasonable to assume that no Chiastocheta flies were involved.
Stroh (2015) cites (Hitchmough, 2003) over the vulnerability of seedlings to slug predation in unmown and tall swards. This has synergy with the 2023 survey at the Bowland population where the vegetation was tall, rank and appearing to be neither mown nor grazed for many years; here almost all fruiting heads were missing. It was surmised that slugs had predated the fruiting heads. Similar decapitated stems were observed in the Yorkshire Dales but to a lesser degree. Indeed, in cultivation I have observed slugs ascending T. europaeus plants in late evening and there being many flower buds missing the following morning. I have encountered a similar problem with dense thatch swards and the decimation of plants of Alchemilla acutiloba (Starry Lady’s-mantle) leading to their loss.
Conclusions
My study strongly suggests the following:
• Populations of T. europaeus have declined in and around the West Pennine Moors and have also done so elsewhere in Lancashire over the last 50 years. This correlates with Stroh (2023). However, the reality may be more profound in that individual populations may be declining within core areas but still extant, so creating a record indicating presence in distribution mapping.
• Further studies are suggested to determine whether the Least Concern status needs adjusting to Near Threatened or Vulnerable.
• Seed should be sown soon after ripening and subjected to winter conditions to facilitate spring germination;
• No advantage was found in chilling the seed for 8 weeks before sowing; indeed such seedlings appeared a week or so later than those sown soon after ripening;
• No germination occurred with seeds stored in dry cool conditions and sown in late winter or early spring; and
• Re-establishment sites need to be appropriately managed such that the sward is not thatchy in order to limit the population of slugs and snails.
• Traditional hay field management involving cut and remove or comparable techniques should be viewed as a form of re-wilding; the walk away re-wilding approach allowing grazing stock to graze as they will, may allow too great a vegetation thatch to develop, to the detriment of T. europaeus
References
Hemborg A, & Despres, L. 1999. Oviposition by mutualistic seed parasitic pollinators and its effects on annual fitness of single and multi-flowered host plants. Oecologia 120: 427–436.
Pellmyr, O. 1989. The cost of mutualism between Trollius europaeus and its pollinating parasites. Oecologia 78: 53–59. Stroh, P.A., et al. (12 authors) 2014. A Vascular Plant Red List for England. Botanical Society of Britain and Ireland, Bristol. Stroh, P.A. 2015. Trollius europaeus L. Globeflower. Species Account. Botanical Society of Britain and Ireland website: bsbi.org/species-accounts. Suchan, T., Beauverd, M., Trim, M. & Alvarez, N. 2015. Asymmetrical nature of the Trollius–Chiastocheta interaction: insights into the evolution of nursery pollination systems. Ecology and Evolution. John Wiley & Sons Ltd. Seedaholic: seedaholic.com/trollius-europaeus-globeflower.html [accessed 11 July 2023].
Acknowledgements
I wish to thank S. J. Martin for is first-hand knowledge of the Belmont Trollis europaeus (Globeflower) population and to Lancashire Environmental Records Network for the supply of historical records for Lancashire. Thanks are due to Ben Hargreaves for arranging funding for a polytunnel through the National Highways ‘Network for Nature’ programme. Additional thanks in consideration of Chiastocheta flies go Philip Brighton and Rob Zloch. A final thank you goes to Karen Rogers for her helpful administrations.
Peter Jepson
pjepsonecology@btinternet.com
Mrs Patty Charlotte O’Brien (Miles) 1817–1891 – The Lady of the Flowers
Mrs Patty Charlotte O’Brien (Miles) 1817–1891
The Lady of the Flowers
DAVID C. RAYMENT
Inthe Appendix to her paper, ‘Charlotte Grace O’Brien (1845–1909), her botanical interests and achievements’, which appeared in British and Irish Botany (7(1): 10–29, 2025), Sylvia Reynolds draws attention to the confusion surrounding the authorship of Wild Flowers of the Undercliff, Isle of Wight (1881), by Charlotte O’Brien and Mr C. Parkinson (a Fellow of the Geological Society of London). As Reynolds states, Charlotte Grace O’Brien is often incorrectly cited as the co-author, instead of another Charlotte O’Brien of whom little is known. It is thought Miss Charlotte Grace O’Brien had never visited the Isle of Wight; but another Charlotte O’Brien, Mrs Patty Charlotte O’Brien, the true co-author of Wild Flowers, had resided there for nearly thirty years. Before then Mrs O’Brien had lived in Brighton, Sussex, and several times she exhibited cut flowers and wild flowers at the Brighton Show. This article introduces Mrs Patty Charlotte O’Brien to the reader and offers an insight into her life, character and botanical interests.
Birth and marriage
Mrs Charlotte O’Brien was born Patty Charlotte Miles at London on 3 December 1817 and was baptised there at the parish church of St Andrew, Holborn, on December 30. She was the daughter of John Miles, then a furniture seller, and his wife, also named Charlotte (www.ancestry.co.uk). The twentyeight year old Patty, however, was living on the east Kent coast at Broadstairs, Isle of Thanet, at the time of her marriage to the Irishman, Patrick O’Brien, at the parish church of St Peter. Patrick was then of Dunoon, Argyle, Scotland. The marriage was reported in the Sun (London, 29 April 1846) and is listed in the June Quarter of the Civil Registration Marriage Index (1846). The marriage was also reported in the Glasgow Herald (27 April 1846)
where Patty’s father is incorrectly named William. Thereafter, she appears only to have been known as Charlotte (except on legal documentation), perhaps because of the similarity of her first name with that of Pat, an abbreviation of Patrick.
The Brighton Show and Little Arthur’s Book of Biography
By 1856, however, Charlotte and Patrick were living at 17 Buckingham Place Road, Brighton. In that year she entered the third running of the Brighton and Sussex Floriculture and Horticultural Show, winning the first prize of £2 under the Cut Flowers category heading of ‘Device’. Under the same heading she achieved further success by winning first prize (£1) for her exhibit of wild flowers, for which she was the only entrant (Brighton Gazette, 12 June 1856). Two years later she won 50 shillings for coming second for cut flowers; and for wild flowers she won 10 shillings for coming third (Brighton Gazette, 16 September 1858). The O’Briens later moved a short distance away to number 20, which is where, in March 1859, Charlotte wrote a preface to her Little Arthur’s Book of Biography. The short preface is shown here in full:
‘The names of the good and great of all ages ought to be as familiar as “Household Words” to every child of nine or ten years of age. The fact that they are not so, has induced the writer to publish this very simple Biographical Text Book, which she trusts will prove as useful to children in general, as it has long been to her own little pupils’.
The book proper is in four columns – name in alphabetical order with a very brief description of the individual, place of birth, date of birth and year of death. The list of names begins with Sir Ralph Abercrombie. Other names include: Archimedes, Sir Richard Arkwright, John Audubon, Beethoven, Tycho Brahe, Chaucer, Edmund Halley, Lord Nelson, Sir Isaac Newton, Linnaeus and William
Mrs Patty Charlotte O’Brien (Miles) 1817–1891 –
Wilberforce, to name only a few. Last on the list is ‘Zwingle, or Zwinglius, the great Swiss reformer and patriot’.
Charlotte continued to exhibit at Brighton and, in 1860, in the third tent on the Lawn at the Pavilion, her contribution was: ‘a harp with flowers most artistically’. She also exhibited ‘a pretty collection of wild flowers, forty varieties in pots, to which a third prize is awarded’ (Brighton Gazette, 13 September 1860).
The O’Briens were still living at 20 Buckingham Place Road at the time of the 1861 census where Patrick is recorded as a teacher who obtained his BA degree at T.C.D. (Trinity College Dublin). Charlotte was a governess. They had six female pupils living with them aged between 11 to 18. For the same year Charlotte’s name is listed in Mair’s Schools List (1861) in the section headed ‘Ladies Private Schools’.
The O’Briens leave Brighton for the Isle of Wight
Charlotte and Patrick, however, left Brighton in 1862 when they moved to Ventnor on the Isle of Wight, which is where they established Roseville School in Grove Road. Even so, Charlotte continued to exhibit at Brighton and in 1864 her wild flowers achieved second prize (Sussex Advertiser, 2 July 1864).
On the 1871 census there were four scholars living at the school; all were boys, so Roseville was not a young ladies’ school as at Brighton. Patrick, who was listed on the 1871 census as ‘Private Tutor’, was a well-respected member of the Ventnor Local Board. He died later the same year at the age of 54. Patrick had Erysipelas, a bacterial infection which began in the face (Hampshire Telegraph, 13 December 1871). It must have been a difficult time for Charlotte. He was buried at the then new cemetery (Founded 1870) at Ventnor which is now managed by the Isle of Wight Council. A committee was soon formed to raise monies for a memorial. A monumental cross made of Wicklow granite was erected over the grave in November 1872 (Hampshire Advertiser, 16 November 1872).
Now a widow, Charlotte continued with Roseville School which undoubtedly took up much of her
time, but her interest in plants did not abandon her. During Christmas 1880, Charlotte wrote about her garden in correspondence to the local press: ‘In my garden I have in full bloom four different kinds of roses, veronicas of every shade, double stocks, French Marguerites, cyclamen, scarlet geranium, primroses, and violets in profusion. Surely we may go further and perchance fare worse …’ (Isle of Wight Observer, 1 January 1881). The piece was published outside her locality and even reached America when quoted in The Free Religious Index (1881), a paper published at 3 Tremont Place, Boston by the Free Religious Association every Thursday at a subscription cost of three dollars per year.
The writing of Wild Flowers and a visit to America Wood
The year 1880 was the same year in which she wrote the preface to Wild Flowers of the Undercliff, Isle of Wight, in collaboration with Mr C. Parkinson, FGS. The book was published the following year. The purpose of the book was ‘to enable temporary residents in the Undercliff to become acquainted with the wealth of wild flowers in the immediate neighbourhood, by pointing out the localities in which the different species may be found, and the time of year when they are in bloom. For the latter purpose a concise Floral Calendar is supplied, giving the names of all the rarer kinds of wild plants which may be successfully sought for in the different months’. The calendar runs from January to August: ‘With the month of August, the reign of summer may be said to end, although in fine seasons many of the above-mentioned plants may still be found in September. Then comes the time of seeds and berries, carrying us through what would otherwise be a bare and desolate time. But there is no such period in nature’.
On August 7, 1884, a few weeks before the end of summer’s reign, Charlotte gave a large picnic at America Wood, near Shanklin. Some travelled independently but others went by train ‘which stopped for the occasion near the woods’. When the forty or so persons had finished their lunch ‘some roamed about the woods gathering flowers
and ferns’, while others danced ‘to the music of a violin’. The piece continues: ‘The white and bright-coloured dresses of the ladies looked most picturesque flitting about amongst the surrounding green. The rays of the departing sun were spreading a rosy glow over the glens, cornfields, and trees as the joyous party sat down to tea, and afterwards started home after a memorable and most enjoyable day’ (Lady’s Pictorial, 16 August 1884).
The passing of the Lady of the Flowers
In 1884 Charlotte too was in her August, about to enter her Autumn, passing away on 25 March 1891 at her Roseville residence as her husband had done nearly twenty years earlier. The 73-year-old was an ‘estimable lady, widely known and respected throughout the Undercliff, and her somewhat sodden demise will be mourned by a large circle of friends’ (Isle of Wight County Press, 13 June 1891). Her estate was administered by her brother, Harry Miles (National Probate Calander, 1891), and the property in which she lived was sold at the Commercial Hotel: ‘This property is beautifully placed with south aspect and sheltered from the east and north winds, commands charming views of the sea and landscape, and is within a few minutes’ walk from the railway station. There is a neat garden well
stocked with trees and shrubs. The lot is leasehold for a term of 999 years from 25 December 1863, at the annual rent of £10’ (Isle of Wight County Press, 1891). The leasehold was sold to a Mr T. A. Worrell for £1,080.
It is unfortunate that Patty Charlotte O’Brien has not been properly recognised for her work which was erroneously attributed to another. The co-author of Wild Flowers of the Undercliff, Isle of Wight, now rests peacefully at the same cemetery as her husband. No doubt some cut flowers are nearby, perhaps some wild ones too.
References
Mair, R.H. (ed), 1861. Mair’s Schools List. National Probate Calendar Index of Wills and Administrations for England and Wales, 1891. (Named as Patty Charlotte O’Brien.)
O’Brien, C. 1859. Little Arthur’s Book of Biography, H. & C. Treacher, Brighton; Hamilton, Adams & Co., London. O’Brien, C. & Parkinson, C. 1881.Wild Flowers of the Undercliff, Isle of Wight, L. Reeve & Co., London.
Reynolds, S.C.P., 2025. Charlotte Grace O’Brien (1845–1909), her botanical interests and achievements. Journal of British and Irish Botany 7(1): 10–29.
The Free Religious Index, 27 January 1881. Free Religious Association, 3 Tremont Place, Boston.
David C. Rayment
botany.rayment@btinternet.com

A postcard depicting Ventnor, Isle of Wight c.1890 where Charlotte O’Brien lived from 1862 until her death in 1891. Retrieved from the Library of Congress, www.loc.gov/item/2002708264. Mrs Patty Charlotte O’Brien (Miles) 1817–1891 – The Lady of the Flowers
The extinct Glenridding Hawkweed Hieracium subintegrifolium discovered in two new sites at Ingleborough, Yorkshire
BRIAN BURROW
Glenridding
Hawkweed Hieracium subintegrifolium
Pugsley is a distinctive species in Hieracium section Tridentata characterised by its long, narrow, scarcely toothed (hence subintegrifolium), hairy leaves with conspicuous venation underneath and its broad, spreading panicle of large, dark flowering heads, and bracts with many microglands as well as numerous simple hairs and a few short glandular hairs (Pugsley, 1948; Sell & Murrell, 2006).
It was first discovered by H.W. Pugsley in limited quantity on grassy banks in Glenridding (v.c. 69) on 14 August 1927 (BM). It was last collected on a riverbank at Gillside on 18 August 1953 by J. E. Raven (E, 3 sheets; MNE, 1 sheet, grid reference NY378169). It has not been seen again despite much searching (Halliday, 1997; Sell & Murrell, 2006; Rich, 2013) so was classified as IUCN ‘Extinct’ (McCosh & Rich, 2018). Since 2013, I have also looked for it and Tim Rich has looked three times when camping at Gillside.
In August 2015, I found a few plants of a section Tridentata species in a side pothole at Pillar Pots, Ingleborough (SD734723). The plant had finished flowering and was difficult to access safely but I collected a few seeds from which I grew three plants and prepared herbarium specimens.
In July 2019, I found a few similar plants on Southerscales limestone pavement on Ingleborough (SD74077632, v.c. 64; Figure 1) which I provisionally identified it as H. subintegrifolium. In 2020, I collected a few seeds from which I grew a couple of plants and made some more herbarium specimens.
In March 2025, specimens from both sites were shown to T. Rich and compared with Raven’s 1953 Glenridding specimens, which, after allowing for cultivation, were found to be a good match for H. subintegrifolium. The cultivated plants were larger with more pronounced toothing on the leaves as might be expected compared to smaller plants with remotely denticulate leaves from Glenridding. This is a significant rediscovery of an extinct species in two new sites with an extension of range. Further surveys are planned for 2025.
Acknowledgements
I would like to thank Mark Lynes for help with field work and use of his photographs and Tim Rich for confirming my identification.
References
Halliday, G. 1997. A Flora of Cumbria. Centre for North-west Regional Studies, University of Lancaster, Lancaster. Pugsley, H.W. 1948. A prodromus of the British Hieracia Journal of the Linnean Society of London (Botany) 54: 1–356. McCosh, D.J. & Rich, T.C.G. 2018. Atlas of British and Irish Hawkweeds (Pilosella Hill and Hieracium L.) (2nd edn).
Botanical Society of Britain and Ireland, Harpenden. Rich, T.C.G. 2013 Surveys of three endemic Lake District Hawkweeds: Hieracium filisquamum, H. fissuricola and H. subintegrifolium. Unpublished contract survey for Natural England, September 2013. National Museum of Wales. Sell, P.D. & Murrell, G. 2006. Flora of Great Britain and Ireland, Volume 4: Campanulaceae–Asteraceae. Cambridge University Press, Cambridge.
Brian Burrow bburrow@hotmail.co.uk

Figure 1. Hieracium subintegrifolium (Glenridding Hawkweed) on Southerscales, Ingleborough (v.c. 64) showing scarcely toothed leaves. Inset, capitulum. Mark Lynes

BEGINNER’S CORNER
Speedwells (Veronica) Part 3
This is the third and final part to our look at the commoner and more widespread speedwells (Veronica spp.) that occur in Britain and Ireland. Here we look at ways to identify the four speedwell species that are commonly found in wetland habitats. While some of the other species may occasionally be found in damp grassland or seasonally damp spots, these are the speedwells that are true denizens of wet places, typically occurring along the margins of rivers, streams, lakes and ponds and sometimes even standing in the water itself. As well as their love for wet places, these species all have another common trait – they have their flowers arranged in long spikes or racemes and it is details of these flower spikes, plus the appearance of the leaves, that will help with identification. When finding a speedwell in a wetland habitat, consider the shape of the leaves first, then move to the flowers: take a careful look at the flower colour, the size of the bract at the base of each flower and check whether the spikes of flowers
Veronica anagallis-aquatica (Blue Waterspeedwell) or its hybrid. Mike Crewe
arise opposite each other in the leaf axils or whether they alternate with each other, arising only from the base of one of a pair of opposite leaves. Note that the leaf toothing is very shallow in these species.
These four wetland speedwells are all rather widespread in Britain and Ireland, though three of the four become scarce or absent in upland areas and in the western and northernmost regions. As with so many of our wetland plants, they tend to do better where water quality is good and a small amount of range contraction has been noticeable in some species, especially in the more heavily populated areas of southern and central England.
As mentioned in Part 2, it is useful to remember that a common stalk that carries several flowers is a peduncle, while the individual stalks of each single flower are known as pedicels.
Mike Crewe mikedcrewe@gmail.com
MIKE CREWE



Brooklime (Veronica beccabunga). This is always a delightful species to find, with its glossy and rotund leaves and rich, royal blue flowers that may be found throughout the summer months. Brooklime is a relatively lowgrowing perennial that spreads to form mats on damp mud and along the margins of waterways, particularly favouring muddy deposits on the meanders of sinuous streams and rivers. It can be found throughout Britain and Ireland except in the highermost regions of Scotland and some of the Outer Hebrides. Amongst this group, Brooklime is easily identified by its rounded, glossy leaves and by the intense blue of its flowers. Flowers May–September. Photos: Mike Crewe



Marsh Speedwell (Veronica scutellata). Bucking the trend of the other wetland species covered here, Marsh Speedwell is local and generally uncommon in lowland England, becoming more frequent to the north and west, while it is the most widespread species in the upland regions of Scotland, Wales and northern England. In Ireland it is probably the most widespread species after Brooklime. Marsh Speedwell is a creeping perennial that can be identified by its relatively small and narrow leaves compared to others in the group (typically 2–4 cm in length) and by the flower spikes, which appear in only one axil of each pair of leaves, typically alternating up the stem. The leaves and stem often have a bronzy tint and the flowers are pale lilac-blue in colour, sometimes almost white. Flowers June–August. Photos: Mike Crewe (top left, top right); John Norton (bottom left).






Blue Water-speedwell (Veronica anagallis-aquatica) and Pink Water-speedwell (V. catenata). These two species are so similar that I have considered them both together; both are generally absent from higher regions of Britain but are otherwise widespread, with Blue being more common in much of Ireland and lowland parts of Scotland. These are both relatively large species, forming small stands of upright stems to 60 cm tall along the margins of a wide range of wetland habitats. They have rather elongate, stalkless, lanceolate leaves, though lower leaves of Blue Water-speedwell can have short petioles. Despite the names, the flower colour can often be difficult to determine, being various shades of bluish-pink or pinkish-blue, but the colour of ‘classic’ plants can be more helpful in identification. To tell the two species apart, it is best to look closely at the racemes of flowers, which appear in pairs opposite each other in the upper leaf axils. Each individual flower has a small leafy bract at its base which in Blue Water-speedwell is shorter than the pedicel of the flower that it is adjacent to. In Pink Water-speedwell this bract is almost as long as or longer than the adjacent pedicel. This feature should be checked when the flower is fully open, as the pedicels can lengthen later as the plant forms seed capsules, when this feature becomes less useful. In addition, the blue species has narrower and more attenuate, acutely pointed sepals, while the sepals of the pink species tend to be more ovate and abruptly (obtusely) pointed.
To complicate matters the hybrid between the two (Veronica × lackschewitzii) is common in some areas and often occurs in the absence of either parent. It usually grows more vigorously and taller than the two species and has longer racemes of flowers (usually >40 flowers). The capsules are often empty or poorly formed, so feel flat when squeezed with the fingers. There is a useful comparison table of characters of the two species and the hybrid in the Plant Crib account, available on the BSBI website (bsbi.org/plant-crib). Flowers June–August. Photos: top left and middle, Blue Water-speedwell (Mike Crewe); top right, Hybrid Water-speedwell (John Norton); bottom: Pink Water-speedwell (Mike Crewe).
ADVENTIVES & ALIENS
Adventives & Aliens News 36
Compiled by Matthew Berry
Flat 2, Lascelles Mansions, 8–10 Lascelles Terrace, Eastbourne, BN21 4BJ m.berry15100@btinternet.com
Anadditional record of Melica ciliata (Silky-spike Melick) was brought to my attention by Ken Balkow after the species featured in Adventives & Aliens News 35 (see v.c. 6). He found it on a green roof at Sharrow School, SK3485 (v.c. 63) in 2010, the plant’s identity having been confirmed by Bruno Ryves. It is published on p. 403 of The South Yorkshire Plant Atlas (ed. G. Wilmore et al., 2011), but was not in the DDb in early 2025.
I have included another of Andy Shaw’s epiphytic Tree-fern aliens in the present compilation (see v.c. 42), the justification for so doing as before. The plant was grown on and identified by him and at the time of writing there are at least three other species isolated in the same manner that await naming. It is inconceivable that this garden centre is the only one to which potentially or proven weedy alien plant species are hitching a ride in this way, or the only one from which they could be unwittingly disseminated by customers.
Claudia Pavia Kaplan, the finder of the v.c. 21 Cenchrus americanus (Pearl Millet) (see Adventives & Aliens News 35) provided two very helpful clarifications concerning her record. Firstly that the plant occurred in a postcode that meant it was more accurately located to Central Tottenham than to Tottenham Hale. Secondly and more importantly, that a more likely origin for the grass than bird seed was food or kitchen waste, the seeds being sold as ‘Bajra’ or ‘Whole Millet Seeds’ in many local Asian shops and markets.
Corrigendum: BSBI News 158, p. 31 (Adventives & Aliens News 34), in the fifth line of Zinnia elegans paragraph – ‘discoid capitula’ should be ‘radiate capitula’.
V.c. 1b (Scilly)
Cotula coronopifolia (Buttonweed). White Island (SV924175), 6/2024, D. Mawer (comm. R. Parslow): a number of flowering plants in a shallow seasonal pool on this uninhabited island off St. Martin’s, the sixth v.c. 1b record (the first having been made in 2002). It was growing with Suaeda maritima (Annual Sea-blite), a species only recorded once before in Scilly (Rosemary Parslow, pers. comm.) (see England roundup, p. 62). The site was revisited in 2025 by James Faulkenbridge when the plants were found to be flowering well in spite of efforts in 2024 to eliminate them. A glabrous, fleshy, procumbent to ascending annual or perennial (Asteraceae) to 30 cm, a native of S. Africa and New Zealand. It has alternate, aromatic, sheathing stem leaves that are basically broadly linear but ranging from entire to deeply lobed, even on the same plant, and solitary, yellow, hemispherical, discoid capitula (c.1 cm across) on long stalks. There are no receptacular scales and

Cotula coronopifolia, White Island, Scilly (v.c. 1b). James Faulkenbridge
the flattened, winged achenes lack a pappus. It is often associated with damp, saline places in some of which it is well naturalised. Adventives & Aliens News 19, v.c. 58. Stace (2019): 799.
V.c. 4 (N. Devon)
Helleborus × hybridus ‘Atrorubens’ (Lenten-rose Hybrid). Tawstock (SS560299), 27/3/2023, R.I. Kirby & S.H. Kirby: spread by seed from grave planting, with one single-flowered plant on a second grave and many first year seedlings in adjacent grassy areas, Tawstock Burial Ground. The first v.c. 4 record. A perennial herb (Ranunculaceae) with deeply lobed bracts, relatively few saucer-shaped flowers and leathery overwintering palmate leaves confined to the base of the stem. It differs from native H. viridis (Green Hellebore) in its larger flowers (5–7 cm across vs 3–5 cm), entirely free follicles (vs fused at base) that are shortly stalked (vs sessile) and its yellowish-green to purplish petaloid sepals (vs pale green). The hybrids are probably more commonly grown in gardens than is the pure H. orientalis (Lenten-rose) from Turkey. The Tawstock plants have been referred to ‘Atrorubens’, a clump-forming cultivar with reddish-purple to purple-pink, nodding or outward-facing flowers. Stace (2019): 112.
Jasminum beesianum (Red Jasmine). Fremington (SS51423250), 17/10/2024, R.I. Kirby & S.H. Kirby (conf. J. Poland): single clump in scrub beside a footpath. The first v.c. 4 record. An often scrambling deciduous garden shrub (Oleaceae) to 2 m, a native of China. The flowers are bright reddish-pink and usually six-lobed but are rather small and few in number in terminal leaf axils. They are followed by black, more or less globose berries (5–12 × 5–9 mm) that are presumably palatable to some birds. The simple leaves (more or less ovate-lanceolate and acuminate) distinguish it vegetatively from yellowflowered J. nudiflorum (Winter Jasmine), which has ternate leaves and white-flowered J. officinale (Summer Jasmine), which has pinnate leaves. J. humile (Yellow Jasmine) with yellow flowers and pinnate leaves, which differ from those of the preceding species in being alternate rather than (sub)opposite, is an occasional garden shrub from Asia that might
be better placed in another genus, Chrysojasminum Adventives & Aliens News 25, v.c. 50. Verbena rigida (Slender Vervain). Barnstaple (SS56663305), 12/9/2024, R.I. Kirby (conf. J.J. Day & M. Berry): single plant growing on a pavement edge where it abutted a grass verge, Firs Grove. The first v.c. 4 record. A tuberous-rooted perennial herb (Verbenaceae) to 60 cm from S. America, with sessile, stiff, hispid, serrate stem leaves and lilac or pale blue flowers arranged in terminal, elongating spikes, each individual corolla c.6 mm across. V. bonariensis (Argentine Vervain) is a taller plant with inflorescences that are more branched, leaves that are not as stiff or as leathery and bracts that are shorter than or equal to the calyx (vs longer than calyx). The fruiting spikes of V. bonariensis are shorter (c.2–3 cm vs greater than 4 cm) and the individual corollas only 3–3.5 mm across. It also has a shorter corolla tube (5–7 mm vs c.9 mm) and a shorter calyx (c.3 mm or less vs greater than 3 mm). In gardens

Verbena rigida, Barnstaple, North Devon (v.c. 4). Bob Kirby
V. rigida is a popular container and bedding plant, when it might behave more as an annual than a perennial. Stace (2019): 651.
Symphoricarpos × chenaultii (Chenault’s Coralberry). Barnstaple (SS57163242), 6/11/2023, R.I. Kirby (conf. M. Duffell): well naturalised by streamside landscaping that has matured into rough scrub, Rose Lane. The first v.c. 4 record. A deciduous, arching, garden shrub (Caprifoliaceae) with stolons that root at the tips. It is the artificial cross of N. American S. orbiculatus (Coralberry) and S. microphyllus from Mexico. S. albus (Snowberry) is a suckering shrub with pure white fruits (vs fruits usually with white spots on pink or pink spots on white) and glabrous styles (vs hairy). It also has hollow, hairless twigs (vs solid and hairy) and more or less glabrous leaf surfaces (vs leaves hairy below). S. orbiculatus has smaller (4–6 mm vs 6–10 mm), uniformly pink fruits and shorter (1–2 cm vs 2–3 cm), more obtuse leaves. S. × doorenbosii (Doorenbos’ Coralberry) is another garden hybrid (S. albus × S. × chenaultii), and the influence of S. albus is observable in the slight lobing of some leaves and the less hairy lower leaf-sides. Adventives & Aliens News 27, v.c. 110.
V.c. 5 (S. Somerset)
Anthyllis vulneraria subsp. carpatica (Kidney Vetch). Taunton (ST25002568), 11/6/2024, S.J. Leach (conf. J. Akeroyd): 12 plants on rough stony bank on east side of Bridgwater Road, Bathpool Bridge. The first Somerset record of this central European perennial herb (Fabaceae). Adventives & Aliens News 30, v.c. 95.
Davidia involucrata Baill. (Dove-tree). Yeovil (ST552162), 22/10/2024, I.P. Green: small tree self-sown near to parent tree. The first Somerset record. A deciduous tree (Nyssaceae) to c.18 m with a finely fissured grey-brown bark; it is a native of south-western China. The alternate, ovate, sharplytoothed leaves are cordate at the bases and abruptly contracted at the tips; they have slightly branched secondary veins, raised below and densely whitehairy lower surfaces. The inflorescences consist of a mass of many male flowers with one to seven stamens each and purple anthers, and an apically
inserted female or bisexual flower, which unlike the male flowers has a perianth. Each inflorescence is enclosed by two very conspicuous, white, wing-like bracts which have clearly inspired the tree’s best known English names: Dove Tree, Ghost Tree and Handkerchief Tree. The three- to six-seeded oblong ovoid fruit has a greenish, pale-speckled outer surface, turning purplish when ripe, 3–4 cm × 1.5–2 cm at maturity. It hangs down on a c.10 cm long stalk. Planted trees that have narrower leaves with shiny, glabrous lower leaf surfaces are sometimes segregated as var. vilmoriniana
Delosperma cooperi (Hook. f.) L. Bolus (Cooper’s Dewplant). Minehead (SS96864689), 13/7/2024, G.E. Lavender: a c.3 m × 0.5 m patch on rocky bank beside road. The first Somerset record. An evergreen, mat- or lawn-forming perennial (Aizoaceae) to c.15 cm tall, a native of S. Africa and a relatively hardy garden plant in Britain. It has trailing stems and fleshy cylindrical pale green leaves that are up to 5 cm in length. The long-lasting daisylike flowers are c.5 cm across and have numerous glossy, purplish-pink ‘petals’ (petaloid staminodes). Another garden plant from S. Africa, Delosperma nubigenum (Schltr.) L. Bolus, has one record in the DDb for v.c. 11 (2005), BSBI News 101, pp. 42–43. It has smaller ovoid leaves and yellow ‘petals’. In the generic key on p. 531 of Stace (2019), Delosperma comes closest to Drosanthemum – the former differs in having long, subulate stigmas (vs short and broadly triangular stigmas) and leaves that lack conspicuous papillae (vs leaves with very conspicuous papillae) (Eric Clement, pers. comm.).
Origanum majorana L. (Pot Marjoram). Minehead (SS9746), 24/7/2024, G.E. Lavender: the first Somerset record of this shrubby, highly aromatic perennial (Lamiaceae) from northern Africa and south-western Asia. Adventives & Aliens News 15, v.c. 15.
Campanula carpatica (Tussock Bellflower). Minehead (SS96584664), 18/6/2024, G.E. Lavender: on wall by pavement. The first Somerset record. A glabrous, clump-forming perennial (Campanulaceae) from the Carpathians that has decumbent stems and longpetiolate, ovate-cordate, sharply toothed leaves, 1.3–
2.5 cm long. The erect solitary flowers, on longish pedicels, can be white, pale blue to bright blue or violet, the shallowly bell-shaped or saucer-shaped corollas c.3 cm across with five very short apical lobes. Two other rockery plants, both from Italy, C. fragilis and C. isophylla Mor., are rather similar but have a less tufted, more trailing habit and flowers that are generally more flattened out and star-like with longer corolla lobes. They also have capsules with basal pores (vs capsules with subapical pores in C. carpatica) and three very short stigmas carried on a very much longer and protruding style (vs three long stigmas that are more or less as long as or perhaps sometimes longer than style). Stace (2019): 707.
V.c. 6 (N. Somerset)
Parthenocissus henryana Graebn. ex Diels (Chinese Virginia-creeper). Pitney (ST44512850), 8/6/2024, Somerset Rare Plants Group: one plant on verge outside village hall. New to Somerset. A deciduous woody climber (Vitaceae) to c.10 m that has fourridged stems and palmate leaves of (3)5(9) leaflets; it is a native of China. Each leaflet is obovate, glabrous to somewhat hairy, has a dark green or bronzy green background colour and silvery or pinkish veining, turning entirely crimson later. As a garden plant it is less rampantly spreading than P. quinquefolia (Virginia-creeper).
V.c. 7 (N. Wilts)
Sorghum halepense (Johnson-grass). Bradford-on-Avon (ST83286116), 29/6/2025, F. Sinclair (comm. K. Newbert): seeded into angle of garden wall and pavement, Woolley Terrace. The first v.c. 7 record. A rhizomatous perennial grass to 1.5 m, a native of northern Africa. It has relatively narrow leaves (1–2 cm wide) and a diffuse, narrowly pyramidal panicle up to 50 cm long. The spikelets are in pairs of one sessile, bisexual spikelet and one stalked, sterile or male spikelet, or in triplets of one sessile and two stalked spikelets. In S. halepense the bisexual spikelet usually has a long bent awn and drops whole at maturity. S. bicolor (Great Millet) is a taller annual with wider leaves (1–7 cm wide), a usually more compact panicle and bisexual spikelets that

Sorghum halepense, Bradford-on-Avon, North Wiltshire (v.c. 7). Fran Sinclair
are usually unawned (sometimes short-awned) and persistent at maturity. Adventives & Aliens News 28, v.c. 55.
V.c. 8 (S. Wilts)
Lythrum virgatum L. (Slender Purple-loosestrife). Westbury (ST86395175), 29/10/2024, D.E. Green & H.J. Crouch (conf. M. Berry): 200 plus plants bank of lake (an old flooded brick pit) and adjacent ecological mitigation area. It is thought to have profusely self-seeded after being introduced as part of the original planting scheme around two balancing ponds adjacent to the old brick pit (David Green, pers. comm.). The first Wiltshire record. An erect, slender, glabrous perennial (Lythraceae) to 120 cm that has lax terminal spikes of rosy-purple flowers, c.1.5 cm across, and narrowly lanceolate
opposite leaves; it is a native of central and eastern Europe and a garden plant in Britain and Ireland. Adventives & Aliens News 30, v.c. 14.
Dipsacus laciniatus (Cut-leaved Teasel). Burbage (SU2260), 17/7/2025, F. Sinclair (comm. K. Newbert): three mature plants close to hedge in wide margin around oat crop on a clay/chalk substrate, Harepath Farm. The first Wiltshire record. An erect prickly Eurasian biennial (Dipsacaceae) to 3 m that has ovoid-cylindrical capitula and connate upper stem leaves. It differs from native D. fullonum (Wild Teasel) in being taller and having many leaves cut at least halfway to the mid-vein (vs leaves at most dentate or serrate). Adventives & Aliens News 19, v.c. 64.

V.c. 10 (Isle of Wight)
Campanula erinus L. (Annual Bellflower). Ryde (SZ603903), 21/5/2025, M. Spencer (comm. C. Pope): six plants at the foot of a retaining wall in the car park of a gardening centre outside Ryde. There were plants with blue flowers and others with white flowers. Mark Spencer thinks that it could have arrived in the soil of container-grown olives imported from Cordoba in Spain and standing in pots immediately above the retaining wall. A few weeks later the plants had withered away but there is a suggestion it has been present as a weed at this site for a few years (Colin Pope, pers. comm.). A branched, slender, bristly hairy annual (Campanulaceae) to 30 cm, a native of Mediterranean Europe. The dainty, short-stalked, tubular flowers are 3–5 mm long, typically with a white corolla tube and five much shorter, erect to spreading, pale blue lobes but it seems the flowers can also be lilac, reddish or completely white; they are borne terminally or in the axils of the stem branches. The calyx is equal in length to the corolla and has five lanceolate teeth which in fruit are spreading and star-like. The petiolate lower leaves are spathulate to c.3 cm long, the sessile upper leaves are obovate and both coarsely toothed. Only one other annual Campanula, C. ramosissima Sm., has appeared in Adventives & Aliens News. Like that other Mediterranean plant recently featured in the column, Veronica cymbalaria, it might have a future as a weed at least in the southern

Campanula erinus, Ryde, Isle of Wight (v.c. 10).
Colin Pope
Dipsacus laciniatus, Burbage, South Wiltshire (v.c. 8).
Fran Sinclair
part of the country. Unlike V. cymbalaria, there do not seem to have been any previous records for Britain or Ireland.
V.c. 11 (S. Hants)
Salvinia minima Baker (Small Salvinia). Gosport (SU59490198), 12/7/2025, J.A. Norton & D.R. Allan: small colony of plants floating at edge of moat with Lemna trisulca (Ivy-leaved Duckweed) and Least Duckweed (Lemna minuta), Fort Brockhurst. This seems to be the first record for Britain and Ireland. A free-floating aquatic annual (possibly perennial in tropical regions) fern (Salviniaceae), a native of S. America and central America. It has a


thin, horizontal, densely hairy, somewhat branched stem up to 20 cm long and leaves in whorls of three – two rounded-elliptic, floating leaves (c. 0.8–1.5 cm × 0.5–1.0 cm, sometimes with a distinct fold in the centre) with 10+ pairs of lateral veins, and one finely divided, submerged leaf which functions as a root. The upper surfaces of the floating leaves are covered in many papillae, each divided at the tip into four spreading multicellular hairs in S. minima. The sporocarps occur at the base of the submerged leaves (although none have been detected in the Gosport population) and are organised in a spike in S. minima (clustered or long-pedicellate in other species). Several Salvinia species seem to be on offer commercially to aquarists, etc., including the European native S. natans (L.) All. and the sterile, potentially highly invasive S. molesta D.S. Mitch. (Giant Salvinia) from S. America (Booy et al., 2015), although misnaming is rife with much material that is not S. natans being mislabelled as such. As already mentioned the hairs at the tips of the papillae on the upper leaf surfaces provide one useful character for separating the species (e.g. hairs united at the tips rather than spreading in S. molesta). Other potentially useful characters include the size and shape of the floating leaves, e.g. large (up to 2 cm × 6 cm) and rounded-ovate in S. molesta, the number of lateral veins (e.g. 15–20 pairs in S. natans), and again, as already mentioned, the way the sporocarps are organised. For more information see onlinelibrary.wiley.com/doi/10.1111/epp.12909 and Schou et al. (2023)
Cardamine occulta Hornem (Cryptic Bitter-cress). Havant (SU730077), 10/6/2024, S. Thomas; Gosport (SU584002), 29/7/2025, J.A. Norton (conf. R. Mabutt). In the first case, abundant in pots at Havant Garden Centre, Bartons Road; and in the second, in several pots in one section growing Clematis at the Alver Valley Garden Centre (John Norton, pers. comm.). JN also visited Havant Garden Centre and noticed that most plants were also in pots of Clematis from the same supplier as the Gosport plants, labelled ‘Grown in the Cotswolds’. The first and second v.c. 11 records respectively. Adventives & Aliens News 34, v.c. 42, etc.
Salvinia minima, Gosport, South Hampshire (v.c. 11).
Debbie Allan (top), John Norton (bottom).
Schizanthus pinnatus Ruiz & Pav. (Poor-man’sorchid). Near Bournemouth (SZ12709140), 22/5/2025, D. Leadbetter (det. M. Berry): two plants coming up in cleared/disturbed ground by Boscombe Overcliff Drive; they were in full flower on 16/6/2025. A rather tender, somewhat variable garden annual (Solanaceae) from Chile. Adventives & Aliens News 31, v.c. 81.

11). David Leadbetter
V.c. 12 (N. Hants)
Solanum nitidibaccatum (Green Nightshade). Near Bordon (SU78133588, SU78123588, SU78173589), 13/8/2024, A.R.G. Mundell & M. Parker: one plant at each grid reference, Slab Common. A prostrate to ascending, white-flowered, glandular hairy annual (Solanaceae) that has rhombic-ovate leaves (3–7 cm long, entire to coarsely toothed) and an often overall yellowish-green appearance. A native of S. America, it is an increasing casual of various kinds of waste ground and also an arable weed, e.g. in Sugar Beet. The fruiting calyx is much larger than the flowering calyx and more or less half-hides the berry which is shiny green to brown at maturity, each one containing up to c.30 seeds. The very similar S. American Solanum sarrachoides (Leafy-fruited Nightshade) favours a subtropical climate (vs more temperate) and is consequently very much rarer in Britain, with many if not all of the early records being errors. In S. sarrachoides the fruiting calyx lobes are at least as long as the berry which contains greater than 50 seeds. It also has
wider corolla lobes (5–7 mm vs 2–4 mm). Clement et al. (2005), p. 216 (as Solanum physalifolium var. nitidibaccatum). Stace (2019): 611.
V.c. 14 (E. Sussex)
Urospermum dalechampii (L.) Scop. ex F.W. Schmidt. Willingdon (TQ60020295), 8/6/2025, M. Berry (conf. E.J. Clement): one plant flowering and fruiting in unmown grass of road verge, Maywood Avenue; self-sown from nearby garden. The first Sussex record. A softly hairy, rosette-forming perennial (Asteraceae) to c.40 cm, a native of Mediterranean Europe. The basal leaves are runcinate (i.e. dandelion-like) with a small terminal lobe and more or less backward-pointing, triangular, lateral lobes; the upper leaves with fewer lobes or entire and clasping the stem. The pale creamy-yellow or lemon yellow ligulate capitula are c.3 cm across (up to c.6 cm across?) and held singly on peduncles that are slightly inflated and easily compressed below the involucres. Each ligule terminates in five tiny often dark-coloured teeth and the outer ligules are often reddish-coloured on the reverse. The phyllaries are in a single row, fused below and narrowly dark-edged. The achenes are beaked and there is a pappus of feathery bristles. There are records from the mid-1990s for v.cc. 1 and 3. It was present in the Keyhaven Marshes area (v.c. 11) from 2000 until at least 2008, and was apparently persistent from 2002 in Lymington, also v.c. 11 (last record in DDb for 2014). Another Mediterranean species, U. picroides, has been recorded as a wool and esparto casual, Clement & Foster (1994). It is a bristly hairy annual with a pure white pappus (vs buff-coloured) and achenes with a distinct swelling at the base of the beak (vs base of beak gradually widening).
V.c. 15 (E. Kent)
Campanula rapunculoides (Creeping Bellflower). Great Stonar (TR32665957), 23/6/2024, K. Chapman: a patch of flowering plants beside Monks Way. A relatively slender upright perennial (Campanulaceae) with a creeping rootstock to c.80 cm, found Europewide as a native and once much more common as a
Schizanthus pinnatus, near Bournemouth, South Hampshire (v.c.
garden plant in Britain and Ireland. It has relatively narrow, regularly toothed leaves and long tapering racemes of nodding, narrowly bell-shaped, bluepurple flowers. The native C. trachelium (Nettle-leaved Bellflower), which can also occur as a garden escape, is hairier, tufted (vs patch-forming), has sharply angled stems (vs stems hardly angled) and erect to erecto-patent calyx-teeth (vs patent to reflexed). The peak decades for this species as a garden escape/ outcast in Kent, as elsewhere, were the 1950s–1970s, and it is more rarely recorded now. The first of two significant alien plant finds made by Ken Chapman in this 1 km square on 23/6/2024, see below. Kent Botany 2024, p. 20. Stace (2019): 710.
Lilium candidum (Madonna Lily). Great Stonar (TR32725953), 23/6/2024, K. Chapman: on the central reservation of Monks Way, near a road crossing. The third v.c. 15 record, the last one having been in 2012. A tall bulbous perennial (Liliaceae) that has variously whorled elliptic stem leaves and terminal racemes of large, white, sweetly scented, more or less horizontally held flowers, a native of the Balkans and south-western Asia. It is not thought to be a deliberate planting at Great Stonar but its mode of arrival is not obvious. Kent Botany 2024, p. 26. Adventives & Aliens News 19, v.c. 59.
V.c. 16 (W. Kent)
Polystichum munitum (Western Sword-fern). Goudhurst (TQ72683786), 23/5/2024, D. Newman: large specimen on a shady roadside bank, Maypole Lane. The first v.c. 16 record and for Kent as a whole. A tufted evergreen one-pinnate fern (Dryopteridaceae) to 1.2 m, a native of western N. America and a garden plant in Britain and Ireland. The leaves are glossy dark green above, serrate with stiff spinulose teeth, and each pinna has an enlarged thumb-like basal lobe that is always on the side closest to the leaf apex. Kent Botany 2024, pp. 39–40. Adventives & Aliens News 32, v.c. 86.
V.c. 17 (Surrey)
Amaranthus blitum (Guernsey Pigweed). Shirley (TQ3765), 28/7/2025, C. Bateman & G. Kitchener (comm. S. Medcalf): found along several streets in

Amaranthus blitum, Shirley, Surrey (v.c. 17).
Jon Wilson
gutters and paving cracks in a residential road, the Spring Park area. The deeply retuse leaves of this procumbent, seemingly increasing annual (Amaranthaceae) are distinctive. Adventives & Aliens News 32, v.c. 27. Clement et al. (2005), p. 63. Stace (2019): 530.
Eragrostis pilosa (Jersey Love-grass). Shirley (TQ3764), 28/7/2025, C. Bateman & G. Kitchener (conf. G. Kitchener/comm. S. Medcalf): in a gutter in the Spring Park area. The only modern v.c. 17 record. An annual grass with glabrous leaf sheaths to 70 cm, a grain, bird seed and wool casual from Eurasia and northern Africa. The open panicle (with erecto-patent branches at maturity), small linear spikelets (c. 3–7 × 1 mm), tiny lower glumes (c. 0.5 mm long) and leaf margins and pedicels without sessile, wart-like glands help to distinguish it from other Love-grasses. Adventives & Aliens News 15, v.c. 11. Stace (2019): 1117.
V.c. 19 (N. Essex)
Lotus hirsutus (Canary Clover). Wix (TM16792848), 27/5/2025, M. Padfield: two plants self-sown in a pavement with no immediately adjacent gardens and the nearest gardens apparently without L. hirsutus
A softly, silvery-hairy Mediterranean subshrub (Fabaceae) to c.50 cm; it has leaves of five obovate leaflets and subsessile clusters of up to c.10 flowers (10–20 mm long), the corollas pinkish or whitish and pink-tinged, the keels dark-tipped (vs all petals yellow in the other Lotus species). It was formerly Dorycnium hirsutum and has pinnate leaves with the five leaflets
all on the rachis and vestigial stipules. The capsule is 6–12 mm long and exceeds the calyx. Mike Padfield noted the plants as being quite fresh-looking in the recent drought conditions. There are 60 records minus duplicates shown in the DDb, with possible naturalisation in some cases, for English v.cc. 6, 9, 11, 13, 14, 15, 16, 21, 24, 26, 28, 29, 34, 37, 39, 53 and 56, from 1993 (v.c. 24) onwards. There is one record for Ireland (v.c. H12) and one for v.c. 113 (Sark). There are presently no records in the DDb for Wales or Scotland. BSBI News 135, pp. 71–72. Stace (2019): 164.
V.c. 21 (Middlesex)
Broussonetia papyrifera (L.) Vent. (Paper Mulberry). Gunnersbury Park (TQ188792), 20/8/2022, D. Cahen (conf. M. Maculan): one seedling in park, seemingly self-sown. The first British and Irish record. A deciduous, dioecious shrub or small tree (Moraceae) to c.15 m, a native of Asia and sometimes grown ornamentally in parks and gardens. The alternate leaves may be unlobed (ovate, serrate) or deeply palmately lobed; they are 15–20 cm long. The male catkins are c.5 cm long. The 2 cm wide female inflorescence is a more or less spherical cluster of greenish flowers sporting very long styles and the infructescence that succeeds it, a 2–3 cm wide cluster of red or orange drupes. The leaves of Morus alba (White Mulberry) and M. nigra (Black Mulberry) can

also be lobed or unlobed and like them, B. papyrifera has twigs and petioles with white latex. However, B. papyrifera suckers strongly (vs not suckering), has leaves that are always very roughly hairy (vs smooth or slightly rough-hairy) and inedible, bristly, orange or red fruits (vs edible, smooth, white, red or black fruits).
The English name and specific epithet commemorate the fact that B. papyrifera has been, and to some extent still is, used in the making of paper. It is known to produce fruit when in cultivation in London (Henry Miller, pers. comm.).
Euphorbia serpens Kunth (Matted Sandmat). Northfields (TQ170790), 1/6/2025, M. Maculan (det. T. Walker): several plants growing along edge of street paving. The first v.c. 21 record. A mat-forming annual (Euphorbiaceae) from tropical America and a container alien. As well as glabrous stems and capsules, it has relatively small leaves (only 2–5 mm long) which are entire and the 1 mm long seeds have smooth surfaces. There are records in the DDb for v.cc. 4 (1991), 34 (2008), 18 (2011), 37 (2014), 10 (2015/2023), 11 (2020), 29 (2023) and 32 (2025).
Adventives & Aliens News 22, v.c. 11.
Kitaibela vitifolia Willd. (Russian Hibiscus). Regent’s Park (TQ282823), 15/8/2021, D. Cahen: single flowering and fruiting plant in an urban park, apparently self-sown. The first British and Irish record. A hairy, white- or pale pink-flowered perennial (Malvaceae) to 2.5 m, a native of the northwestern Balkan Peninsula and very occasionally in gardens. The flowers are c.5 cm across, the petals blunt, somewhat notched, well-spaced and fused only at the extreme base. The leaves are serrate and three- to five-lobed with the terminal lobe often much the longest. It has an epicalyx of five ovateacuminate segments, versus three broadly ovate segments in Malope, which like Kitaibela, has the mericarps organised in an ovoid mass known as a schizocarp (vs in a ring in Alcea, Malva and Althaea). The epicalyx segments are linear in Hibiscus and the fruit is a capsule. The generic name is often misspelt ‘Kitaibelia’.
Gilia capitata (Blue Thimbleflower). Brentford (TQ183781), 25/5/2023, D. Cahen: growing in long grass in an urban area, possibly from scattered
Broussonetia papyrifera, Gunnersbury Park, Middlesex (v.c. 21). Daniel Cahen
seed. The are no other v.c. 21 records in the DDb. It was subsequently found in a similar situation c.2 km away. An erect, much branched annual (Polemoniaceae) to c.75 cm, a native of western N. America and a bird seed casual and garden escape in Britain and Ireland. It has variously pinnately divided leaves and blue flowers in dense more or less spherical heads. The corollas have lobes that are much longer than the tube; the stamens are as long as to somewhat longer than the corolla lobes and have glabrous filaments. Gilia achilleifolia Benth., from California, has less densely capitate flower heads, larger corollas (10–20 mm vs 8–12 mm) of a darker blue colour and the stamens are shorter than or at most equal to the corolla lobes. Plants that are not readily assignable to one or other species may occur. The garden annual Gilia tricolor Benth. (Bird’s-eyes), from western California, has lilac, purple-eyed corollas, c.1.5 cm across, in loose, fewflowered corymbs. Stace (2019): 544.
V.c. 28 (W. Norfolk)
Verbascum phoeniceum (Purple Mullein). Dereham (TF98611325), 7/6/2025, A. Prendergast (det. M. Crewe/comm. S. Pryce): self-seeded into pavement crack from adjacent garden. A usually unbranched perennial herb (Scrophulariaceae) to 1 m, a native of south-eastern Europe. The leaves are ovate-oblong, irregularly crenate and often more or less glabrous, the basal leaves being shortly petiolate and up to c.12 cm long, the stem leaves smaller and sessile. The stem has gland-tipped hairs, particularly above, and there is a rather open raceme of violet or bronzepurple flowers, each held on a slender c.2 cm long pedicel. The corolla is 2.5 cm across, the anthers are uniformly reniform and all the filaments have violet hairs. There is one flower per bract axil. The pure species seems to be grown in gardens where the trend was once more towards highly floriferous, sterile hybrids of it. It is also a bird seed alien. Adventives & Aliens News 30, v.c. 9.
Calendula arvensis (Field Marigold). Croxton (TL873867), 22/3/2025, S. Pryce (det. A. Prendergast/comm. S. Pryce): many seedlings in cracks in a house’s paved parking area. A somewhat
stickily glandular hairy, procumbent to ascending (or even erect) annual (Asteraceae) up to 80 cm tall; a native of southern Europe and for the most part a rare casual garden escape in southern England. It has solitary, orange-yellow radiate capitula, 1–2.5 cm across, and clasping, grey-green, oblonglanceolate leaves, entire to shallowly toothed, 3–7 × 1–2 cm. The common annual to perennial garden escape, C. officinalis (Pot Marigold), has larger (7–12 × 1.5–3 cm), bright green leaves and capitula that are 3–7 cm across with ligules more or less twice as long as the phyllaries (vs less than twice as long). According to some accounts it also differs in having erect fruiting heads (vs pendent) and achenes that are more consistently spiny and boat-shaped (vs achenes that are usually more variable in form); but these characters are probably less reliable. Clement et al. (2005), p. 346. Stace (2019): 811.
V.c. 42 (Brecs)
Crassula decumbens (Scilly Pigmyweed). Brecknock (SO171375), 2025, A.G. Shaw: growing as a weed on trunk of Dicksonia antarctica (Australian Tree-fern) at The Old Railway Line Garden Centre, Three Cocks. A decumbent to ascending annual (Crassulaceae) to 12 cm, a native of S. Africa and Australasia. It has connate, very acute, linear-lanceolate leaves and solitary axillary four-petalled flowers on 4–10 mm long pedicels, each petal c.2–4 mm long. C. helmsii (New Zealand Pigmyweed) is aquatic (vs terrestrial),

Crassula decumbens, Brecknock, Breconshire (v.c. 42). Andy Shaw
perennial, has longer stems and flowers with petals that are longer than the sepals (vs petals as long as or shorter than sepals). The terrestrial native C. tillaea (Mossy Stonecrop) has more or less sessile, usually three-petalled flowers and shorter (1–2 mm vs 3–8 mm), obtuse leaves. The native C. aquatica (Pigmyweed) also has more or less sessile flowers with petals longer than the sepals. The English name reflects its persistence as a bulbfield weed in v.c. 1b. Clement et al. (2005), p. 137. Stace (2019): 145.
V.c. 55 (Leics)
Isatis tinctoria (Woad). Arnesby (SP616923), 17/4/2025, S.F. Woodward (det. G. Hall from photo): three shoots on the road side of a high wall, clearly self-sown. The first modern (post-1933) v.c. 55 record. The blue-green, sagittate leaves, with a prominent white mid-vein, and sturdy pruinose stems are distinctive; the spreading compound corymbs of tiny yellow flowers and winged, pendent, pale brown fruits (c.2 cm long, flattened and turning purple-brown) even more so. It is widely naturalised in Europe, much rarer and usually impermanent in Britain and Ireland. Adventive & Aliens News 30, v.c. 28.
V.c. 57 (Derbys)
Ornithopus sativus (Serradella). Upper Loads (SK31386992), 18/5/2025, M. Lacey (conf. T.C.G. Rich): about a dozen plants, c.12–15 cm tall, in a field of Trifolium incarnatum (Crimson Clover) and T. hybridum (Alsike Clover) where the crop had failed and rain had washed away soil; a presumed impurity of the clover crop. An annual (Fabaceae) from southwestern Europe, persistent as an introduction in v.cc. 1 and 2 and a rare casual elsewhere. It could be confused with robust plants of O. perpusillus (Bird’sfoot). O. sativus is more upright, has bracts that are half as long as the flowers (vs equalling or exceeding flowers), longer corollas (6–10 mm vs 3.5–4.5 mm) and longer calyces (3.2–5 mm vs 2.3–2.8 mm); it often also has fewer flowers per inflorescence (2–5 vs 3–8), more leaflets per leaf (up to 37 leaflets vs up to 27 leaflets) and straighter fruits. The other recent records in the DDb are for 2008 (v.c. H11), 2011 (v.c. 43) and 2020 (v.c. 12). It was observed growing in
a field as part of a forage crop in Storrington (v.c. 13) in 2025 (Nick Sturt, pers. comm.) and as part of a sown seed mix in Connemara (v.c. H16), also 2025 (Richard Milne, pers. comm.). Stace (2019): 165. Notobasis syriaca (L.) Cass. (Syrian Thistle). Astwith (SK43546449), 1/6/2025, M. Lacey: two plants in two separate fields, in a crop mainly of Phacelia, Trifolium incarnatum, Vicia sativa (Common Vetch) and Onobrychis (Sainfoin). There are no other v.c. 57 records in the DDb. A robust, stiffly erect, usually little branched, annual thistle with a cobwebby indumentum, a native of the Mediterranean region

Ornithopus sativus, Upper Loads, Derbyshire (v.c. 57). Mick Lacey

Notobasis syriaca, Astwith, Derbyshire (v.c. 57). Mick Lacey
and south-western Asia. The leaves are glabrous above and have spiny toothed margins, the upper ones with narrower lobes and amplexicaul. They are marked with conspicuous white veins, very like those of Silybum marianum (Milk Thistle). The erect capitula are c.2.5 cm across and occur singly or in clusters at the end of the stem and stem branches. There is a central plume of numerous purple flowers and an involucre of appressed (but spreading at the tips), overlapping, ovate, usually purplish phyllaries; each capitulum is enclosed by about two to five strongly spine-tipped, overtopping upper leaves. A monotypic genus close to Carduus and Silybum, it differs in the dimorphic pappus – of feathery hairs for the inner achenes and of simple hairs for the outer achenes (vs uniformly of simple hairs in Carduus and Silybum). The maximum height quoted for N. syriaca seems to be around 150 cm; the v.c. 57 plants exceeded this by some 65 cm (Mick Lacey, pers. comm.). Listed as a grain casual in Clement & Foster (1994), it occurred in ‘modified grassland’ in v.c. 23 in 2021 and in a barley field in v.c. 5 in 2023, BSBI News 153, pp. 62–63 (where there is a good summary of past records). It was seen new to Norfolk in similar settings in 2025 (Suki Pryce, pers. comm.).
V.c. 62 (N.E. Yorks)
Rumex thyrsiflorus Fingerh. (Thyrse Sorrel). Redcar (NZ59492517), 21/6/2025, J. Harding-Morris (det. J. Akeroyd): one c.140 cm tall plant on top of narrow embankment on ridge running east from Rainbow Park, Coatham Marsh. This bank was originally sown with a ‘wild flower’ mix which probably included this plant. On a subsequent visit to the site by another local botanist a second plant was found (Dave Barlow, pers. comm.). There is a v.c. 21 record in the DDb (2022); there are also confirmed records for v.cc. 11 and 17. Adventives & Aliens News 32, v.c. 11.
V.c. 68 (N. Northumb.)
Sedum spathulifolium ‘Cape Blanco’ (Colorado Stonecrop). Rothbury (NU056018), 28/6/2024, M.J. Crawley: self-sown garden escape in Back Crofts and Providence Lane. A glabrous, procumbent,
evergreen perennial (Crassulaceae) with rooting stems, a native of N. America and a garden plant in Britain and Ireland. The obovate leaves (0.8–2 cm long) are thick but with definite upper and lower surfaces and distinctly white-bloomed; the yellow, five-petalled flowers (c. 15 mm across) occur in small terminal clusters. This cultivar forms a wide mat or mound to 10 cm tall. The N. American S. oreganum is similar but its leaves are not white-bloomed and its petals are keeled (vs unkeeled). Stace (2019): 148.
Ophiopogon planiscapus Nakai ‘Nigrescens’ (Mondo Grass). Rothbury (NU056018), 28/6/2024, M.J. Crawley: self-sown garden escape in Back Crofts and Providence Lane. A tufted, evergreen, shortly rhizomatous perennial (Asparagaceae) to 20 cm, a native of Japan and popularly grown for ground cover in gardens. The linear, grass-like leaves are all basal and the lilac or purplish-white flowers are borne in a raceme on a flattened scape. There is a petaloid perianth of six free tepals, and a semiinferior, three-celled ovary; the fruit is a berry. In this cultivar the leaves turn ‘black’ (actually very dark purple). The genus was once placed in Haemodoraceae, ‘The Blood Root Family’, along with such genera as Wachendorfia
V.c. 69 (Westmorland)
Allium siculum (Honey Garlic). Bouth (SD3286), 13/5/2023, E.F. King, W. Nelson & D.G. Benham; Arnside Parish (SD45207815), 2/6/2024, D. Shaw; Arnside (SD458780), 19/5/2025, P. Whitehead: High Road track to Arnside Knott, away from habitation (Paul Whitehead, pers. comm.). The first, second and third v.c. 69 records (the second and third being within the same monad) of this bulbous perennial garden plant (Amaryllidaceae) from southern Europe. The subspecies does not seem to have been noted. Adventives & Aliens News 27, v.c. 64, etc.
V.c. 83 (Midlothian)
Verbascum phlomoides (Orange Mullein). North-east of Shawfair (NT32237018), 29/7/2025, H. Kinnin (det. M. Crewe): on an area of waste ground that is now being developed. The first v.c. 83 record. A densely
woolly hairy biennial herb (Scrophulariaceae) to 2 m that usually has unbranched inflorescences (although not in this case), a native of Europe. The large orangey-yellow corollas and non-decurrent stem leaves separate it from V. thapsus (Great Mullein). The Turkish V. bombyciferum (Broussa Mullein) has lower stamens with proximally white-hairy filaments (vs subglabrous in V. phlomoides) and smaller anthers (up to 4 mm long vs greater than 4 mm). Stace (2019): 640.
Bromus secalinus var. hirtus (Rye Brome). Bonnington Mains (NT12236959), 12/7/2025, S. Jury (det. M.P. Wilcox): quite a lot on a field margin. The only other recent v.c. 83 record of the species is for 2024. At maturity, the slow-to-break-up spikelets (12–20 mm long), furrowed caryopsis and exposed rachilla distinguish it from other Bromus. An annual grass to 1.2 m, found Europe-wide, that is increasing as a weed of crops and casual of waste ground after a marked decline. This variant has softly hairy spikelets (vs glabrous in var. secalinus). Stace (2019): 1091.
V.c. 96 (Easterness)
Francoa appendiculata Cav. (Bridal Wreath). Cawdor (NH847499), 26/5/2025, L. Parkerson: well established on a wall within castle grounds, Cawdor Castle. The first v.c. 96 record. An evergreen perennial herb (Francoaceae, formerly in Saxifragaceae) to 1 m, a native of Chile and grown as a garden plant. The leaves are all basal with a large toothed terminal

lobe and a few much smaller lateral lobes that divide the rest of the leaf more or less to the midrib. The flowering stems are not or little branched, the 1.5–2.0 cm wide flowers in long racemes, each flower with four white or pink entire petals. The fruit is a four-celled capsule. For the sake of simplicity I have taken the current view that Francoa is a monotypic genus containing one variable species, F. appendiculata, the other combinations (see below) being synonyms. There are four records for Francoa in the DDb. Two for v.c. 1 (F. ramosa), where the first British record was made at Tresco in 1993; one for v.c. 66 (F. ramosa) in 2011; and one for v.c. 6 (F. sonchifolia) in 2019. F. appendiculata was also found on the inner harbour wall at Dartmouth (v.c. 3) in 2015, BSBI News 130, p. 48.
Erica × darleyensis (Darley Dale Heath). Dallaschyle Wood (NH82084765), 28/3/2025, A. Amphlett & E.C. Amphlett (conf. M.J. Crawley): a small plant on south verge of track in plantation. The first wild v.c. 96 record. An evergreen more or less decumbent shrub (Ericaceae) to c.60 cm (but often much shorter) that flowers from early winter through to early summer; it is the artificial cross of E. erigena (Irish Heath) and the south-central European E. carnea (Winter Heath). It has purplishpink or white corollas that are c.6.5 mm long with at least partly exserted anthers (perhaps sometimes included?). The dark green leaves (4–8 mm long) are 4 (5)-whorled and strongly revolute with very little of the leaf undersides left showing. The ridges running down the stem from each leaf base fade or narrow obviously before reaching the next leaf down. The Dallaschyle plant had exserted anthers and glabrous stems and might have originated from a nearby house or been introduced as a result of vehicle movements/track construction and/or repairs (Andy Amphlett, pers. comm.). Stace (2019): 562.
Muscari latifolium J. Kirk (Broad-leaved Grapehyacinth). Gartally area (NH48813069), 2/4/2025, A. Amphlett: several plants in flower on a roadside bank under trees, beside the minor road to Gartally. The first v.c. 96 record. The first British record was in 2004 for v.c. 94 and was also made by Andy Amphlett (pers. comm.). A bulbous perennial (Asparagaceae)
Francoa appendiculata, Cawdor, Easterness (v.c. 96).
Louis Parkerson
garden plant to 20 cm from Turkey. It has fewer leaves per bulb than M. armeniacum (Garden Grapehyacinth) (1–2 vs 6–18) and the leaves are wider (1–3 cm vs 0.5–0.9 cm) and more hooded; the contrast between the pale blue, upper sterile flowers and the blue-black, lower fertile flowers is more marked in M. latifolium. There are now around 50 records, the majority for v.cc. in Scotland and the north of England, with few or none for the southwest or south-east of England. There are no records in the DDb for Ireland.
V.c. 104 (N. Ebudes)
Sidalcea hendersonii (Henderson’s Checkermallow).
Waterloo (NG66032312), 27/7/2023, S.J. Bungard (det. M.J. Crawley): in coastal roadside scrub. There is also a 1987 v.c. 104 record for S. hendersonii. A N. American genus, Sidalcea are collectively erect perennial (sometimes annual) herbs (Malvaceae) to c.1.5 m. They have spike-like racemes of hollyhocklike flowers that lack an epicalyx, have linear stigmas, outer stamens with branched filaments and a fruit of one-seeded carpels in a ring, They typically have upper leaves that are more deeply, palmately lobed than the lower. The plants grown in British and

Irish gardens seem often to be complex hybrids/ cultivars rather than pure or ‘typical’ species. S. hendersonii differs from S. malviflora in lacking its hairy upper leaf surfaces and having smooth nutlets (vs reticulately-veined nutlets in S. malviflora ). The Waterloo plants also lack the deep red flower colour of Sidalcea ‘Shetland Red’ or Sidalcea ‘Croftway Red’ (Mick Crawley, pers. comm. to Stephen Bungard). In his determination Mick Crawley has left open the possibility that the Waterloo Sidalcea might be a hybrid. There are 24 records for S. hendersonii in the DDb. A species additional to those in Stace (2019), S. neomexicana A. Gray, was recorded in v.c. H3 in 1986 (det. N.K.B. Robson), Reynolds (2002). Stace (2019): 404.
Centaurea montana ‘Amethyst in Snow’ (Perennial Cornflower). Fiscavaig (NG33973421), 13/5/2025, J. Walmisley (comm. S.J. Bungard): three plants in grassland on roadside verge. The first v.c. 104 record of the cultivar and tenth of the species. A rhizomatous perennial herb (Asteraceae) to 80 cm that has decurrent oblanceolate leaves, winged stems and usually blue, sweet-scented, pseudoradiate capitula; a mountain species native to central and southern Europe which is a very popular garden plant and frequent garden escape. In this cultivar the capitula have white outer ‘petals’ and purplish centres. Clement et al. (2005), p. 294. Stace (2019): 736.
References
Booy, O., Wade, M. & Roy, H. 2015. Field Guide to Invasive Plants and Animals in Britain. Bloomsbury, London & New York. Clement, E.J. & Foster, M.C. 1994. Alien Plants of the British Isles. Botanical Society of the British Isles, London. Clement, E.J., Smith, D.P.J. & Thirlwell, I.R. 2005. Illustrations of Alien Plants of the British Isles. Botanical Society of the British Isles, London.
Cope, T. & Gray, A. 2009. Grasses of the British Isles, BSBI Handbook No. 13. Botanical Society of the British Isles, London.
Poland, J. & Clement, E.J. 2020. The Vegetative Key to the British Flora (2nd edn). John Poland, Southampton.
Reynolds, S.C.P. 2002. A Catalogue of Alien Plants in Ireland. National Botanic Gardens, Glasnevin.
Schou, J.C. et al. (8 authors). 2023. Aquatic Plants of Northern Europe and Central Europe including Britain and Ireland Princeton University Press, Princeton.
Stace, C.A. 2019. New Flora of the British Isles (4th edn). C & M Floristics, Middlewood Green, Suffolk.
Sidalcea hendersonii, Waterloo, North Ebudes (v.c. 104). Stephen Bungard
Alien plants in the greater Dublin area in 2024
SYLVIA REYNOLDS
In October 2023 Julian Reynolds and I downsized to an apartment overlooking Dublin Bay at Salthill near Dun Laoghaire on the east coast of Ireland. To regain some sort of normality after the move, we started to record species growing ‘in the wild’ in our new area in early 2024. What became most noticeable that spring was the abundance of Erigeron floribundus (Bilbao Fleabane; including overwintering leaf rosettes) and Polypogon viridis (Water Bent) with inflorescences, particularly on roadsides. Later, Lactuca serriola (Prickly Lettuce) was also common, the three species often occurring together or near each other.
With my long-standing interest in alien plants in Ireland, we decided to survey a suite of more recent arrivals in the greater Dublin area. The five target species selected were E. floribundus, P. viridis, L. serriola, Senecio inaequidens (Narrow-leaved Ragwort) and E. sumatrensis (Guernsey Fleabane).
The earliest Irish record of E. floribundus (as Conyza bilbaoana) was a 1984 Dublin city specimen in the National Herbarium, originally named as ‘Conyza canadensis’ and redetermined in 1996 after such plants had been found near the port at Rosbercon in Co Wexford 1992–1996 (Reynolds, 1997). The closely related E. sumatrensis was first recorded in Ireland at Dublin Port in 1988, and Polypogon viridis in Dublin city in 1998. The first Irish records for Senecio inaequidens were for a few plants in 1999 near Newtownards, Co Down, and near Larne Harbour, Co Antrim; but not reported from Dublin until 2011 at Irishtown ‘Reserve’ (R. McMullen, pers. comm. 2011; now Irishtown Nature Park) and also at Bray, Co Wicklow, in 2012 (T. Curtis, pers. comm. 2012). Modern records of Lactuca serriola date from 1996 at Dublin Port. There are two earlier records of it in Ireland, 1903 on the North Bull in Dublin and 1904 from a hen run in Co Kildare, where it was introduced with imported grain. Unless otherwise
credited, the source for the dates of first records above is Reynolds (2002).
The 2024 survey was carried out at 56 sites on 36 days between 2 July and 7 November, usually with Julian. The sites, nearly all urban and suburban, were spread across the greater Dublin area mainly within or near the M50 ring road, and a number of outlying places were also visited, including in Cos Wicklow and Kildare (Figure 1, Table 1). Sites varied greatly in size, from the commercial centres of neighbourhoods such as Dalkey, Clondalkin and Killester, to larger areas such as the city centre west and east of O’Connell Street and Dublin Port. It was often convenient to park at shopping centres or take the DART (suburban train), then we walked around for an hour or two, also checking adjacent residential roads. We recorded the presence of the target species, their habitats, relative abundance and whether they were in flower and/or fruit.
Notes were made too on any other aliens of interest – casuals and several longer established aliens, particularly Hirschfeldia incana (Hoary Mustard), Rapistrum rugosum (Bastard Cabbage), Senecio viscosus (Sticky Groundsel) and S. squalidus (Oxford Ragwort). Well established and widespread alien species and common weedy species were not normally recorded, e.g. Hordeum murinum (Wall Barley), Mercurialis annua (Annual Mercury), Buddleja davidii (Butterfly-bush) and Centranthus ruber (Red Valerian). The results of the survey are presented below and changes in the alien flora in the greater Dublin area over the last few decades are discussed.
Observations on the target species in 2024
The distribution of the five target species in the greater Dublin area is given below and summarised in Table 1. Details of relevant records have been submitted to the BSBI’s database. Abundance
ADVENTIVES & ALIENS: Alien plants in the greater Dublin area in 2024
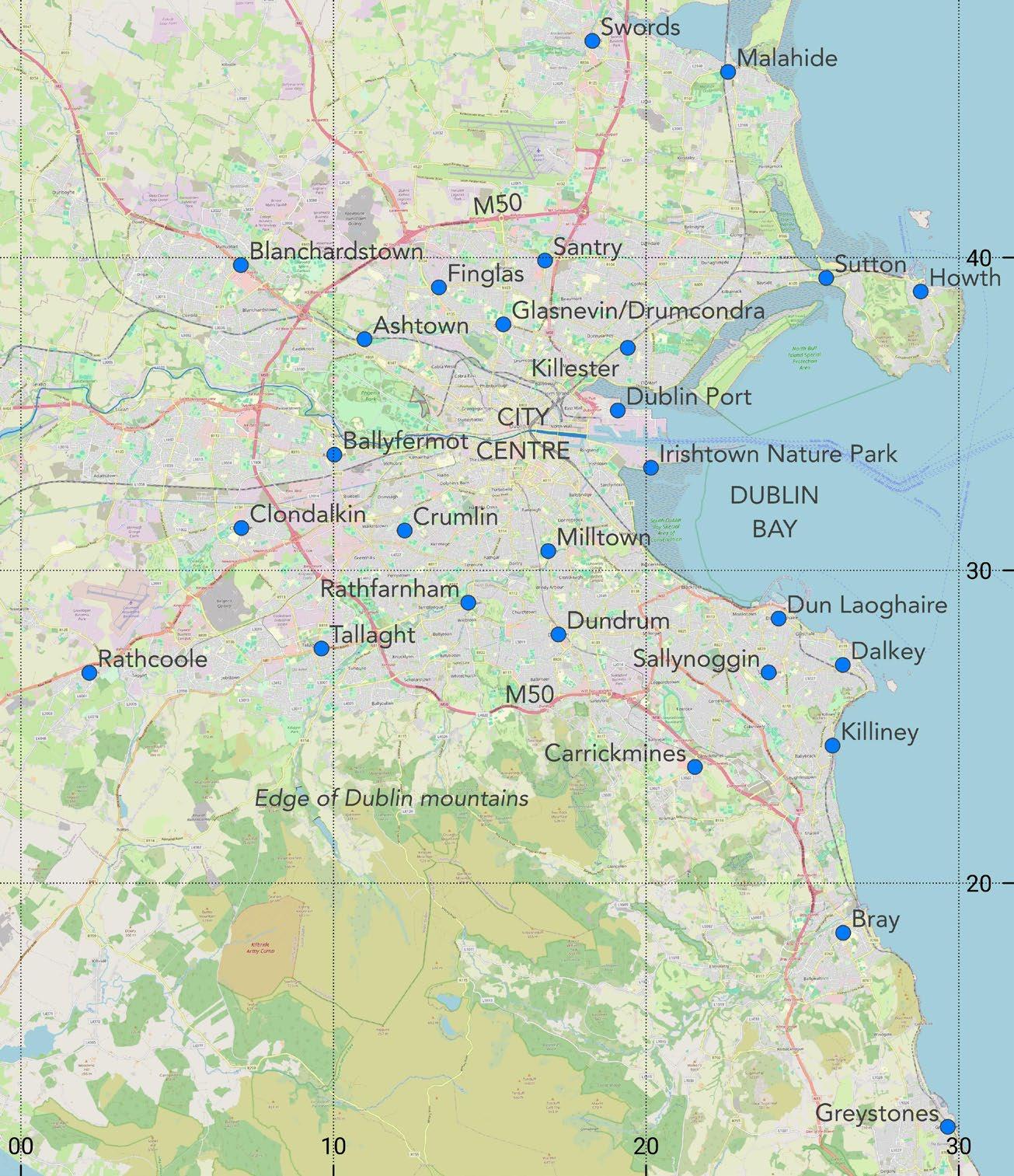
Figure 1. Map of the greater Dublin area showing approximate positions of 28 of the 56 sites visited and 10 km grid lines. See Table 1 for grid references of sites. Map data from OpenStreetMap under the Open Database Licence (openstreetmap.org/copyright).
varied greatly depending on habitat, and there were noticeably fewer and smaller plants later in the season where there had been rigorous cleaning of roadsides and pavement edges, and even of walls.
The two most widespread species were Erigeron floribundus and Polypogon viridis, found at 54 and 49 of the 56 sites respectively, both identifiable all year round. Annual E. floribundus can grow to over
Table 1. Sites visited in greater Dublin area in 2024 (Co Dublin H21 unless otherwise noted), and occurrence of the target species at each site. Details of records submitted to BSBI’s database (DDb).
Ef = Erigeron floribundus; Pv = Polypogon viridis; Ls = Lactuca serriola; Si = Senecio inaequidens; Es = Erigeron sumatrensis
Site names
Dublin city and port
Dublin Port area (including Tolka Estuary Greenway)
Pigeon House Road/ Ringsend
Irish grid ref(s) Target species
O1835, O1935, O2034, O2035 Ef Pv Ls Si Es
O1833, O1933 Ef Pv Ls Si
Irishtown Nature Park O1933 Ef
Liffey quays, N side (including North Wall Quay) O1634, O1734 Ef Pv Ls Si
Liffey quays, S side O1634, O1734 Ef Pv
City centre E of O’Connell Street (including George’s Dock, Spencer Dock and Connolly Station)
O1634, O1635, O1734 Ef Pv Ls Si Es
City centre W of O’Connell Street O1434, O1534 Ef Pv Ls Si
City centre, Westland Row to St Stephen’s Green O1533, O1633 Ef Pv
Heuston Station O1334 Ef Pv Ls
Sites within and near M50 ring road
Ashtown O1037 Ef Pv
Ballyfermot O0933 Ef Pv
Ballymun O1539 Ef Pv Ls
Blackrock station O2129 Ef Pv Ls Es
Blanchardstown O0639, O0739 Ef Pv Ls
Booterstown O2029, O2030 Ef Pv Ls
Cabinteely O2325 Ef Pv
Carrickmines, near shopping centre O2123 Ef Ls
Clondalkin
O0631 Ef Pv Ls
Crumlin, near hospital O1231 Ef Pv Ls
Crumlin, at/near Sunshine Industrial Estate O1232 Ef Pv Ls
Dalkey
O2626 Ef Pv
Dundrum O1628, O1727, O1728 Ef Pv Ls
Dun Laoghaire
O2328, O2428 Ef Pv Ls
Finglas O1339 Ef Pv
Glasnevin/Drumcondra O1537 Ef Pv
Harold’s Cross O1431 Ef Pv
Kilbarrack O2238 Ef Pv
Killester O1937 Ef Pv Ls Es
Killiney station and shore near station O2524, O2624 Ef Pv
Kimmage O1331 Ef Pv Ls
Kylemore Road, just N of Grand Canal O1033 Ef Pv Ls
Milltown O1630 Ef Pv Ls Es
Monkstown/Salthill O2328 Ef Pv Ls Si
Palmerstown O0834, O0835 Ef Pv
Ranelagh O1631, O1632 Ef Pv Ls
Rathfarnham Shopping Centre, near O1328 Pv Ls
Sallynoggin O2326, O2426 Ef Pv Ls Es
Sandymount O1833 Ef Pv Ls Si
Santry O1639 Ef Pv Ls
Shankill O2521 Ef Pv
Stillorgan Shopping Centre, near O1927 Ef Pv Ls
Tallaght O0827, O0927 Ef Ls
University College Dublin O1829, O1830, O1929 Ef Pv Ls Si
Outlying sites
Bray, Co Wicklow, N of Dargle River near shopping centre (H21 N of Dargle River) O2618, O2619 Ef Ls Si
Bray, Co Wicklow, S of Dargle River (H20) O2618, O2718 Ef Pv Ls Si Es
Greystones, Co Wicklow (H20) O2912 Ef Pv
Howth village and harbour O2839 Ef Pv Si Lucan O0335 Ef
Malahide O2246 Ef Pv
Maynooth, Co Kildare (H19) N9337 Ef Pv
Newcastle O0028 Ef
Rathcoole O0226 Ef Pv
Sutton, shore O2639 Ef
Sutton station, near O2539 Ef Pv Si
Sutton, St Fintan’s Cemetery O2738 Pv
Swords O1846 Ef Pv
ADVENTIVES & ALIENS: Alien plants in the greater Dublin area in 2024
150 cm high, and it was starting to flower in early July, producing fruits by mid-August, and still flowering and fruiting into at least early October. The leaves of overwintering rosettes are dark shiny green and coarsely toothed. This species occurred as scattered plants or in dense stands, most commonly along pavement edges (which includes roadside kerbs) and along the bases of walls, on walls themselves, and in open or waste ground. It can compete in uncut rough grassy places and was also found in paving cracks, neglected gardens, flower/shrub beds, at the bases of trees and lampposts, and by railways. A few plants were seen at the top of stony shores at Bray and Killiney, while there were numerous plants among characteristic seashore species and garden escapes or discards on the shore near houses at Sutton.
In contrast, Polypogon viridis is a low-growing annual or short-lived perennial grass. Plants were flowering in the spring, then flowering or with goneover inflorescences over the summer, and even into December at Monkstown. It grew most commonly along pavement edges and bases of walls, sometimes with weedy species such as Capsella bursa-pastoris (Shepherd’s-purse), Polygonum aviculare (Knotgrass) and Stellaria media (Common Chickweed); also in paving cracks and occasionally forming dense patches as at Emmet Square, Blackrock. It was less common as scattered plants in flower/shrub beds, and was surviving among graves in the cemetery near Sutton where herbicide had been used.
Annual or biennial Lactuca serriola at 31 sites was a fairly regular component of the assemblage with E. floribundus and P. viridis. Vegetative plants were first seen in June, then starting to flower by early July, and with flowers and fruits by mid-July. The whole plants were going over in September, and new young plants were noted again in mid-October and early November. L. serriola mainly occurred as scattered plants, but occasionally formed dense stands as on waste ground just north of the river in Bray and by Beach Road, Sandymount. Plants grew to about 150 cm in open ground and were usually much shorter along pavement edges and bases of walls. It was less often found in pavement cracks, on walls, around lampposts and trees, or in flower/
shrub beds. Large plants were conspicuous by the railway at Clontarf station.
Senecio inaequidens, recorded at 12 sites, is a wintergreen perennial, distinctive with its yellow flower heads and narrow leaves. It was flowering by at least mid-June, over the summer and into early November. This species was most abundant in the Dublin Port area, growing in dense patches on open ground, also by some roads in the port and by the newly opened Tolka Estuary Greenway (for walkers and cyclists) bordering the port. It was found near the port – on East Wall Road and in paving cracks on North Wall Quay, and across the River Liffey by Pigeon House Road. Many plants were seen in the open ground of dried-out George’s Dock, and otherwise scattered at pavement edges in the city centre. It was scarcer further out from the city, for example, a solitary plant at University College Dublin, in a planter and on dumped soil at Bray, near the station at Sutton and at Howth harbour. After a spell of very cold weather in early January 2025, some bushy plants of S. inaequidens at Dublin Port were almost completely brown and dead-looking with green leaves only at their base, but nearby were small healthy plants.
Annual Erigeron sumatrensis was much less frequent than E. floribundus, found at 7 of the 56 sites. It was easily distinguished with its less dense inflorescences, slightly larger flower heads, pubescent bracts and particularly the grey-green leaves which are velvety to the touch – as are the leaves of its overwintering rosettes (not seen in 2024). E. sumatrensis had immature flowers in early July, but fruiting plants were not noted until October. It was only found in small numbers: in disturbed ground at Dublin Port and on a pavement by East Wall Road, in open ground at Spencer Dock, near the Mermaid Theatre and at a pavement edge in Bray, in the car park at Blackrock station, on a high wall at Milltown, one in a flowerbed at Sallynoggin and by shops at Killester.
The only casual alien species of interest encountered were a few Phalaris minor (Lesser Canary-grass) and one plant of Echinochloa crus-galli (Cockspur) in new flowerbeds by the greenway at Dublin Port, several Laphangium luteoalbum (Jersey Cudweed) in paving cracks on North Wall Quay, and
ADVENTIVES & ALIENS: Alien plants in the greater Dublin area in 2024
patches of Galinsoga quadriradiata (Shaggy Soldier) beside George’s Dock, on Westland Row and a lane off Nassau Street in Dublin city, and near Pound Lane in Maynooth, Co Kildare.
Observations on other alien species of interest
To get an idea of any changes in the more permanent Dublin alien flora, notes were made in 2024 on a number of longer-established species, all known in Ireland since at least the 19th century.
Hirschfeldia incana (at 9 of the 56 sites) and Rapistrum rugosum (at 8 sites) occurred at some of the same sites or separately. H. incana was seen in open ground at Dublin Port and by Pigeon House Road, on waste ground at Spencer Dock and in a planter near Sherriff Street in the city centre. There were occasional plants of it and R. rugosum in the new flowerbeds by the greenway bordering the port and both species grew by the coastal path in Irishtown Nature Park, not far from the port where they have been known since the early 1980s. Elsewhere, both species were found sparingly at University College Dublin and near the railway at Salthill and Dun Laoghaire. R. rugosum was recorded in disturbed ground where ‘wild flowers’ had been sown near Rathfarnham Shopping Centre, at the edge of a playing field at Sutton, on the shore at Sutton where there was much Erigeron floribundus, and also noted in grass verges beside IKEA near Ballymun, while H. incana was seen with other aliens in open ground at Ballymun and a few plants with E. floribundus at the top of the stony shore at Killiney.
Senecio viscosus (at 8 sites) was scarce in the city centre, only seen on a small piece of waste ground west of O’Connell Street, at Heuston and Connolly stations, by the railway at Booterstown and Dun Laoghaire, and further south in the car park near Greystones station; also by shops at Killester and at Clondalkin. S. squalidus (at 5 sites) was scattered in the city centre, including by the railway between Connolly and Grand Canal stations, and by Pigeon House Road and at the edge of Dublin Port by East Wall Road. Neither of these species were found in the main port area.
Other species noted included Melilotus officinalis s.s. (Ribbed Melilot) at Dublin Port, by Pigeon House Road and in nearby Irishtown Nature Park, and M. altissimus (Tall Melilot) at the edge of Blackrock Park near the railway and by the water at Bissett’s Strand, Malahide. There were several patches of Solanum nigrum (Black Nightshade) at Dublin Port, also found by Pigeon House Road and Sherriff Street in the city. Hordeum murinum is still widespread in the greater Dublin area, Sisymbrium orientale (Eastern Rocket) occasional in the city centre, Mycelis muralis (Wall Lettuce) in the city centre and found at more sites south of than north of the River Liffey, while Mercurialis annua did not seem as common in the city as formerly.
Discussion
Detailed surveys, with good habitat descriptions, of Dublin’s inner city flora 1979–1981 (Wyse Jackson & Sheehy Skeffington, 1984) and of Co Dublin mainly in the 1980s and early 1990s (Doogue et al., 1998), as well as specific accounts of alien plants in Ireland (Reynolds, 2002), are the baselines used to check what changes have occurred in the flora of the greater Dublin area in recent decades. Most of the alien species under discussion are widely distributed in the world outside their native ranges (POWO, 2025; Stroh et al., 2023).
Of the longer-established aliens, Hirshfeldia incana was not mentioned in the Flora of Inner Dublin (Wyse Jackson & Sheehy Skeffington, 1984) as it was not recognised at the time and so recorded in error as Brassica nigra (Black Mustard) (Rich, 1988). Under the latter name it was described as occurring on waste ground, often attaining a large population size and usually found with Rapistrum rugosum which was considered firmly established. These two species in the 1990s were particularly common on waste ground in built-up areas, but rare elsewhere in Co Dublin. Senecio viscosus was only found at two sites in the city in 1981, and had spread and was occasionally found in large populations by the 1990s, while S. squalidus was described as one of the commonest weeds in the inner city and still a common urban weed in the 1990s. By 2024, these four species were noted usually
only in smaller numbers where they occurred at the sites visited; even Hordeum murinum and Mercurialis annua (both characteristic of the Dublin flora) and weedy species such as Sisymbrium orientale appeared less frequent than formerly. The wealth of derelict sites scattered throughout most parts of Dublin city was commented on by Wyse Jackson and Sheehy Skeffington (1984). Some explanations for the decline since then of these and other species include that there are now fewer derelict sites and areas of open ground, that mechanical cleaning of streets and pavement edges is definitely more thorough, and herbicide used, for example, along the railways.
One of the surprises during our survey was not to find, except for a small number of garden escapes, any alien species new to us – not even some casuals at Dublin Port. It is likely that there was less spillage during unloading and transporting than formerly and herbicide was widely used, or maybe there were less seed contaminants in the cargoes. Of species recorded at the port in the late 1980s and 1990s, over half (mostly rare casuals) were not seen again. Only a single plant of Echinochloa crus-galli and a few of Phalaris minor were found in flowerbeds at the port. These species are well established in Britain and apparently only becoming so more recently in Co Wexford in the south-east of Ireland, including as arable weeds (Green, 2022). Two other casuals found in Dublin city, Galinsoga quadriradiata and Laphangium luteoalbum, have a limited distribution elsewhere in the country – the latter very common at and near Rosslare Harbour.
As might have been expected from informal observations over the last few years, the more recent arrivals Erigeron floribundus and Polypogon viridis were found to be widespread and locally abundant in 2024, both having become well established in the greater Dublin area. Lactuca serriola occurred with these species at over half of the same sites, while Senecio inaequidens and E. sumatrensis were less frequently encountered.
Erigeron floribundus is a distinctive species with its hispid stems, usually glabrous phyllaries, disc flowers with a 5-lobed corolla, coarsely serrate rosette leaves, etc. It was first recognised in Britain and at the
Rosbercon/New Ross port in Co Wexford in the mid-1990s, but it took a while for field workers to separate it from better-known E. canadensis (Reynolds, 1997; including descriptions of the species). So, in the Dublin Naturalists’ Field Club’s Flora of County Dublin (Doogue et al., 1998), it was noted that some records of E. canadensis (as Conyza canadensis) may refer to E. floribundus (as C. bilbaoana). While I can stand over my records of the two species, they continue to be confused and some Irish records of E. canadensis in the BSBI’s DDb are doubtful or even erroneous.
Erigeron canadensis, first recorded at Dublin Port in the mid-1930s, was not seen during our survey. However, there are quite recent records in the BSBI’s database for both E. floribundus and E. canadensis by M.P. Wilcox and B.A. Tregale on the same day in 2017 at the Ferry Terminal in Dublin Port. Although 1984 and 1985 were the earliest dates for records of E. floribundus in Dublin, it was not recorded again until 1996, no doubt a later introduction, at Heuston Station and in the city centre – but not at Dublin Port where it was looked for that year (only E. canadensis and E. sumatrensis there). The then BSBI specialist in alien plants, Eric Clement, noted that this species could not withstand drought and he predicted a rapid spread in Ireland (Reynolds, 1997). This rather unprepossessing species was freely seeding and already establishing itself in the city in the early 2000s (Reynolds, 2002). Like E. floribundus, E. sumatrensis is also native to South America. It is clearly different with its softly hairy cauline and rosette leaves, etc., and still much less widespread and less frequent in Ireland, perhaps because it flowers later in the season and needs drier conditions; records are reliable for this species. A fourth species, E. bonariensis (Argentine Fleabane), is a more recent arrival, for example, in the southeast at Rosslare Harbour (Green, 2022) and in Co Down in the north-east (G. Day, pers. comm. 2024), but not yet reported from Dublin. Leaney (2017) discusses problems with the identification of these four ‘Conyza’ species in England.
Polypogon viridis has a wide native range and is widely naturalised elsewhere, but it is not known how it was introduced into Ireland. It seeds prolifically
nearly all year round and has spread rapidly along and near roadsides since first recorded in Dublin city in 1998. The other target species seem to have arrived with cargoes through the ports: E. floribundus at Rossbercon/New Ross, where it was originally identified as Conyza canadensis and was already established there by 1992 (and still there in 2024); E. sumatrensis by 1988 and Lactuca serriola (including native to mainland Europe) by 1996 through Dublin Port; and Senecio inaequidens (native to South Africa) perhaps through Larne Harbour in Co Antrim by 1999, and probably also through Dublin Port. There may well have been later introductions at other ports. In Stace and Crawley’s Alien Plants (2015), they considered the two Erigeron species and S. inaequidens to be ‘transport aliens’, for example, likely to have arrived in Britain from northern France where they were already naturalised. Their wind-blown fruits and those of L. serriola would make dispersal easy. These five species are all now widespread in Britain, particularly in the southern half (Stroh et al., 2023).
In the mid-1930s, Howard Hudson (an active member of the DNFC) recorded plants in the Dublin Port area, including aliens (Reynolds & Nash, 2001), but the port was not included in the inner city survey (Wyse Jackson & Sheehy Skeffington, 1984). Alien plants were surveyed there over several years by this author in the late 1980s and 1990s (e.g. Reynolds, 1996; 2002). With permission, I recorded on the stone-paved quays (resurfaced with tarmacadam in 1993) where grain and animal feed were unloaded, and in open ground and along roadsides. Over 70 species were identified, many of which were casuals originating from seed contaminants and rarely found away from the port. Erigeron canadensis and E. sumatrensis were found there since 1988, both forming large populations for several years, but E. floribundus was not seen there until 1999 and was already becoming more noticeable in 2000. Hirschfeldia incana was abundant and Rapistrum rugosum common too at that time (R. rugosum was inadvertently omitted from the 1996 published table of species).
In 2024 Dublin Port was treated as one large site, visited four times during our survey, but without
access to the quays as there had been in the past. Herbicide was used along nearly all the roadsides and medians, so most existing vegetation was on undeveloped or currently unused open ground behind fences. The widespread escapes Buddleja davidii and Centranthus ruber and the usual weedy species were noted, while E. floribundus and Senecio inaequidens were the most abundant of the five target species, in places with Lactuca serriola and Hirschfeldia incana , and more rarely with Melilotus officinalis, Foeniculum vulgare (Fennel), Avena fatua (Wild-oat) or Triticum sp. (Wheat). A number of aliens, including E. sumatrensis and R. rugosum, were seen sparingly among the exotic ‘wild flowers’ in flowerbeds by the new greenway bordering the port. Polypogon viridis was very scarce in the port area.
To give wider context to the target species surveyed, their relative distributions in Ireland (Stroh et al., 2023) are summarised as follows: Erigeron floribundus is the most widespread (in 177 hectads), particularly in the east, south-east and south; Polypogon viridis (81 hectads) mainly in the east, south-east and around Cork; Lactuca serriola (51 hectads) centred in the east around Dublin; Senecio inaequidens (46 hectads) scattered in the north-east, east and south; and E. sumatrensis (24 hectads) mainly in the east, south-east and around Cork.
Nearly all the sites checked in the greater Dublin area were urban or suburban, where a large proportion of the flora is made up of non-native plants, including garden escapes. Such artificial habitats have some obvious constraints to plant survival, and so the speed of growth and volume of seeds produced would give a competitive advantage. The diversity of alien species was noticeably less at the outlying sites away from Dublin city. Of the target species, Erigeron floribundus, which could be considered an invasive, is capable of establishing itself in quite a variety of habitats, perhaps replacing less competitive species. Lactuca serriola can form tall dense stands too, but it is less frequent and less widespread. At present Polypogon viridis is thriving on its own or with common weedy species, especially along kerbs and pavement edges before they are cleaned. Senecio inaequidens, the most recent arrival,
Alien plants in the greater Dublin area in 2024
was abundant at Dublin Port and found mainly in the city – and it is likely to continue to spread. Although E. sumatrensis seemed to be doing well at Dublin Port after it was first noted in 1988, by 2024 it only occurred very sparingly there; elsewhere it was seen in small numbers too, at scattered sites and not clearly establishing itself.
At present none of the target species appear to be a threat in semi-natural habitats away from built-up areas in Dublin and its surroundings. Only time will tell how the distribution and abundance of these species will settle or be modified, and undoubtedly there will be new arrivals – of course all affected by changing weather patterns too.
Acknowledgements
Julian Reynolds was an integral part of this project and I am very grateful for all his help. I would also like to thank John Norton for kindly preparing the map.
References
Doogue, D., Nash, D., Parnell, J., Reynolds, S. & Wyse Jackson, P. (eds) 1998. Flora of County Dublin. The Dublin Naturalists’ Field Club, Dublin. Green, P. 2022. The Flora of County Wexford. Paul Green, New Ross, County Wexford.
Leaney, B. 2017. Common problems with identification in Conyza: Norfolk experience. BSBI News 135: 7–17.
POWO 2025. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published online at: powo. science.kew.org [accessed March 2025].
Reynolds, S.C.P. 1996. Alien plants at ports and in coastal habitats on the east coast of Ireland. Watsonia 21: 53–61. Reynolds, S. 1997. Conyza bilbaoana also in Ireland. BSBI News 74: 44–46.
Reynolds, S.C.P. 2002. A Catalogue of Alien Plants in Ireland National Botanic Gardens, Glasnevin.
Reynolds, S.C.P. & Nash, D.W. 2001. Howard J. Hudson’s ‘Flora of Alexandra Road District, Dublin, 1933 to 1936’. Irish Naturalists’ Journal 26: 437–445.
Rich, T.C.G. 1988. Hirschfeldia incana (L.) Lagreze-Fossat present in Ireland. Irish Naturalists’ Journal 22: 531–532.
Stace, C.A. & Crawley, M.J. 2015. Alien Plants. The New Naturalist Library. HarperCollins, London.
Stroh, P.A., Walker, K.J., Humphrey, T.A., Pescott, O.L. & Burkmar, R.J. 2023. Plant Atlas 2020. Botanical Society of Britain and Ireland, Durham & Princeton University Press, Princeton and Oxford.
Wyse Jackson, P. & Sheehy Skeffington, M. 1984. Flora of Inner Dublin. Royal Dublin Society in association with The Dublin Naturalists’ Field Club.
Sylvia Reynolds sylviacpreynolds@icloud.com

Julian Reynolds overtopped by Erigeron floribundus (Bilbao Fleabane) at Ashtown in September 2024.
Sylvia Reynolds

Sorbus incana (Silver Whitebeam)
on Brownsea Island (v.c. 9), new to Britain and Ireland
DAVID LEADBETTER
Brownsea
Island in Poole Harbour, Dorset is owned by the National Trust, with the northern half of the island being leased to the Dorset Wildlife Trust who employ two wardens to manage it with the help of volunteers. The wardens live in a building known as ‘the Villa’, now often referred to as the ‘Wildlife and Wetland Centre’ (originally built in the 19th century as a vicarage, but never used as such). Behind the Villa lies St George’s Hill, consisting of open woodland growing over an ancient Pleistocene gravel terrace lying over Branksome Sand.
In January 1990 several hundred Pinus sylvestris (Scots Pine) blew down on St George’s Hill in a notable storm. At the time it was regarded as a disaster, but within a few years regeneration of mostly self-sown Betula (birch) was taking place, which remains the dominant species. Other species growing there include Castanea sativa (Sweet Chestnut), Sorbus aucuparia (Rowan), Quercus ilex (Holm Oak), Corylus avellana (Hazel), Arbutus unedo (Strawberry-tree) and Pteridium aquilinum (Bracken)
Sorbus incana (Silver Whitebeam) in fruit on Brownsea Island, Dorset (v.c. 9), October 2024. David Leadbetter
as well as Pinus sylvestris. Nearby, in Venetia Park (an area where exotics were planted in the 19th century) there are several specimens of Sorbus torminalis (Wild Service-tree).
In the autumn of 2023 I found an unusual Sorbus species growing by a path on St George’s Hill and sent a sample to Dr Tim Rich (the BSBI Sorbus referee), who identified it as Sorbus mougeotii (Mougeot’s Whitebeam). Sorbus mougeotii is a rare native of Belgium, the Alps and the Pyrenees. It is occasionally planted in the UK as a street or garden tree and appears to be naturalised in a few places. The record for Brownsea was apparently a first for Dorset.
In late October 2024 I was doing some recording on Brownsea and decided to have another look at the Sorbus mougeotii. While examining it, I noticed
another, smaller Sorbus growing nearby. Assuming it was the same species, I took a closer look, only to discover it was different, with very distinctive leaves with acuminate tips and large red fruits. I collected a sample and later that day went through the Sorbus Handbook (Rich et al., 2010), but without finding a match for the little tree.
I initially e-mailed some photographs of the species to Tim Rich, who said he did not recognise it. After consulting German literature without success, Tim then circulated my photographs to various European colleagues. In the meantime I collected more material to send to him. The response from Tim’s European colleagues was inconclusive and after my material arrived, he visited the National Museum of Wales to compare my specimen with known hybrids of S. aria × torminalis , but the Brownsea tree did not match any of them.
After more research, Tim finally found a good match in a specimen taken from a tree cultivated in Copenhagen Botanical Gardens since 1968 and labelled as ‘Sorbus incana’ (Silver Whitebeam). Hedlund (1901) gave a brief description of a tree of this name with a half-leaf illustration as having been introduced to the Uppsala Botanical Garden in Sweden from Hamburg in Germany before 1832. He stated: ‘… it stands between S. torminalis and S. aria (Common Whitebeam), but is noticeably closer to the latter. Regarding the leaf shape it resembles S. latifolia (Broad-leaved Whitebeam) the most, but the leaves are thin on the upper side, felted, and the styles are fused at the bottom. The pollen and fruit formation are very poor … apparently a hybrid (aria ×? torminalis) or a variety (descendant) of such.’
Unfortunately, there is no original material in either Hedlund’s herbarium or in Hamburg. The tree in Copenhagen Botanical Gardens came from a nursery, and trees from a graft of the Copenhagen specimen were planted in Alnarp, Sweden in the 1970s and are now widely available in cultivation in Sweden. They are also apparently sold in nurseries in the UK. The neotype for this species has therefore been selected from the Copenhagen tree (Rich & Houston, 2025).



Leaves and fruits of Sorbus incana (Silver Whitebeam). David Leadbetter
The question arises of how Sorbus incana arrived on Brownsea Island. A week after discovering it, I found two Sorbus aria (Common Whitebeam) in the same area, so with Sorbus mougeotii, it meant there were three different whitebeams growing within about ten metres. The initial suspicion was they they might have been deliberately introduced.
However, none of the species appears to have been planted, which I made a point of checking with two previous Dorset Wildlife Trust wardens, going back 40 years. The odd angle that Sorbus incana is growing at reinforces the idea that the tree arrived naturally. The mostly likely source would seem to have been a flock of thrushes passing through one autumn, perhaps having feasted on the berries of Sorbus trees planted in a large garden or park somewhere on the mainland near Poole. A second possibility, though less likely, might be that the Sorbus incana (which is a smaller tree than the other two species present) arrived spontaneously as a result of a cross between one of the Sorbus aria and the S. torminalis growing in Venetia Park.
The Sorbus incana tree on Brownsea is currently the only specimen known in the wild in the UK. The site on St George’s Hill is not open to the public, but can be shown on request and will occasionally be visited on a guided plant walk. The tree will feature
An
in the second edition of the Sorbus Handbook, which will hopefully be published before Christmas 2025.
Acknowledgement
I would like to thank Dr Tim Rich for his time and painstaking research into tracking down Sorbus incana and for reading through a draft of this note.
References
Hedlund, T. 1901. Sorbus Kongliga Svenska Vetenskaps-Akademiens Handlingar, Monographie der Gattung, nov. ser. 35(1): 1–147.
Rich, T.C.G., Houston, L., Robertson, A. & Proctor. M.C.F. 2010. Whitebeams, Rowans and Service Trees of Britain and Ireland. BSBI Handbook No. 14. Botanical Society of the British Isles in association with National Museum Wales, London. Rich, T.C.G. & Houston, L. 2025. New names in British Sorbus (Rosaceae). British and Irish Botany 7(1): 30–36.
David Leadbetter davidleadbetter295@gmail.com
overlooked non-native variant of
Primula veris (Cowslip) originating from wildflower seeds and plantings
DAVID BROUGHTON
Primula veris (Cowslip) is a well-known and popular spring flower that has experienced a widespread historic decline within Britain due to land use change, followed by an upturn in fortune more recently. This upturn is at least in part due to ‘wildflower’ sowings and plantings (Richards & Sanford, 2020). As with many similar introductions these are likely to include stock of non-British origin. In this case, in addition to the native subsp. veris we also seem to have widely occurring plants that conform to the more robust Primula veris subsp. macrocalyx (Bunge) Lüdi. This is a plant native to Eastern Europe and Asia (POWO, 2025).
My understanding and interest in subsp. macrocalyx arose from finding a small colony on restored former
colliery land at Mickletown (v.c. 63), where I have known it and watched it gradually increase for a period of 10 years. This population first caught my eye because of the uniformly tall plants (up to c.20 cm) with atypically large flowers and calyces. Occasionally, particularly where there is more soil moisture or nutrition, larger plants of subsp. veris can be found with flower sizes approaching those of subsp. macrocalyx; however, these lack the distinctive calyx that gives the latter subspecies its name. The calyces are often described as inflated, and as can be seen in the photographs accompanying this article are broadly campanulate in comparison with the more parallel-sided calyces of subsp. veris. Since my original record, I have found it scattered on former
colliery land in my local area and more widely in Yorkshire; most recently as a planting on a rural road verge opposite a cottage, and more surprisingly a lone plant, presumably via seed carried from a garden, by the River Ribble near Settle (both in v.c. 64).
Sell & Murrell (2014) identified the potential for non-native races of Primula veris to occur in the wild, providing details for subsp. columnae and subsp. canescens. However, no reference is made by the authors to subsp. macrocalyx and, despite active searching, I am yet to find the Sell & Murrell taxa. There seems to be good potential for subsp. macrocalyx to occur widely in Great Britain given the frequency of nature conservation sowings and plantings, and also because a brief internet search reveals that this plant is well known in cultivation. This is perhaps not too surprising given its larger stature and ‘larger-than-normal’ flowers make it both a more impactful specimen for the garden, and a much easier target for seed harvesting for commercial purposes.
The merits of subsp. macrocalyx as a discrete taxon have long been debated. For example, a multivariate analysis by Länger & Saukel (1993) found that subsp. macrocalyx could be clearly distinguished within the analysis based on the calyx dimensions, but they remained uncertain of the validity of the taxon and its separation from subsp. veris. Interestingly though, they also found no support for subsp. canescens as a discrete entity.
Schmidt-Lebuhn et al. (2012) revisited this using molecular sequencing (nuclear Internal Transcribed Spacer (ITS) and plastid), and their paper is also notable for its clear, reliable photograph of subsp. macrocalyx next to one for subsp. veris. This study found no apparent genetic differentiation between subsp. veris and macrocalyx but offered no comment on the validity of the subspecies. So in this case, an inability to differentiate is not necessarily the same as no difference. There are a number of plant genera where comparable techniques have returned low success rates in separating species (Yao et al., 2010), including other Primula (Yan et al., 2015), as well as animal taxa, as noted by Falk (2025) in relation to nomad bees with distinct morphologies and ecologies.
Given this, it would seem premature to discount the subspecies, particularly as it has a likely value for tracking and documenting non-native occurrences of Primula veris, and as evidence for the consequences of the current era of widespread habitat creation through sowing and planting of stock of unreliable or unchallenged origin.
For those of a similar mind, the main identification feature to be relied on is the calyx as illustrated in the accompanying photographs. From ten measurements of my local colony made during spring 2025 I recorded calyx lengths of (13)17–20 mm versus a width of 12–17 mm, giving a mean length to width ratio of 1.3 (range 1.1–1.5).
Ten comparable measurements for subsp. veris gave a calyx length of 15 mm and a width of 7.5–8.5 (10.5) mm, and a mean length to width ration of 1.8 (range 1.4–2.0). These measurements of calyx width (discounting the outlier values in parentheses) are broadly consistent with those summarised in Brys & Jacquemyn (2009), albeit with some of my width measurements of subsp. macrocalyx a little narrower.
Flower size is also useful if it is remembered that exceptionally there can be an overlap at the lower end of the range for subsp. macrocalyx. I measured the mean diameter of ten flowers of subsp. macrocalyx to be 16.3 mm (range (14) 17.5–21 mm) versus 10 mm for subsp. veris (range 7–12 mm). Leaf hair length also appears to have some merit but it is harder to measure and I am less certain of its usefulness, with subsp. macrocalyx hairs being 0.3–0.5 mm and subsp. veris no more than 0.3 mm. These measurements (discounting the outlier values) are also consistent with those stated in Brys & Jacquemyn (2009).
Plants worth closer examination often stand out as subsp. macrocalyx because of their taller and more robust nature, and also because the flowers are presented more or less horizontally and therefore are more easily appreciated (another reason for its garden merit) as opposed to the generally more pendent inflorescences of subsp. veris
Currently subsp. macrocalyx is not available as an option within the BSBI Recording App, so recorders will need to record under the species and note this subspecies in the comment box. A clear photograph ADVENTIVES & ALIENS: An


Primula veris subsp. macrocalyx at Mickletown (v.c. 63). Top: whole plant with iPhone case for scale; bottom: close-up of the large forward-facing flowers and distinctive calyx. David Broughton


should also be provided to support third party verification of records.
References
Brys, R. & Jacquemyn, H. 2009. Biological Flora of the British Isles: Primula veris L. Journal of Ecology 97(3): 581–600.
Falk, S. 2025. New bees on the block. British Wildlife 36(5): 320–327.
Länger, R. & Saukel, J. 1993. Systematics of Primula veris (Primulaceae). Plant Systematics and Evolution. 188: 31–55.
POWO 2025. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published online at powo. science.kew.org [accessed 25/05/2025].
Richards, A.J. & Sanford, M. 2020. Primula veris L. in BSBI Online Plant Atlas 2020 , eds P.A. Stroh, T.A. Humphrey, R.J. Burkmar, O.L. Pescott, D.B. Roy, & K.J. Walker. plantatlas2020.org/atlas/2cd4p9h.94t [accessed 25/05/2025].
Top: inflorescences of Primula veris subsp. veris (two stems on left) and subsp. macrocalyx (three stems on right); bottom: flowers of Primula veris subsp. veris (top row) and subsp. macrocalyx (bottom row), showing differences in flower and calyx dimensions. David Broughton
Schmidt-Lebuhn, A.N., de Vos, J.M., Keller, B. & Conti, E. 2012. Phylogenetic analysis of Primula section Primula reveals rampant non-monophyly among morphologically distinct species. Molecular Phylogenetics and Evolution 65(1): 23–34.
Sell, P. & Murrell, G. 2014. Flora of Great Britain and Ireland, Volume 2: Capparaceae-Rosaceae. Cambridge University Press, Cambridge.
Yan, H-F. et al. (7 authors) 2015. DNA barcoding evaluation and its taxonomic implications in the species-rich genus Primula L. in China. PLoS ONE 10(4): e0122903.
Yao, H. et al. (11 authors) 2010. Use of ITS2 region as the universal DNA barcode for plants and animals. PLoS ONE 5(10): e13102.
David A. Broughton
Vice-County Recorder for Mid-west Yorkshire (v.c. 64) vc64bsbi@gmail.com
A new BSBI website designed for our botanical community
SARAH WOODS
The BSBI website is being brought up to date, with new and improved features. A small team of staff has been working alongside members and with a web designer on this monumental task, and below we outline the hows and whys of the task at hand, and what members can expect from the new, refreshed website on launch and into the future.
Why refresh the website?
In early 2025, we hosted exploratory meetings with a representative selection of members, including several Vice-County Recorders to look at the current site and think through how it could better meet everyone’s needs. For a while it had been clear that the site was ‘ageing’, becoming more difficult to use as well as maintain. We also gathered first impressions from individuals who hadn’t used our website before, and laid out the technical issues that a redevelopment would allow us to address.
These initial discussions were invaluable. In the end a number of practical and collective reasons were identified that drove the decision to overhaul the existing BSBI website – the majority of which were raised by users in those first meetings:
1. It had become difficult to find relevant resources quickly:
(a) The homepage had become ‘dated’, ‘unfocused’ and ‘cluttered’ – not the first impression of the Society we wanted to give.
(b) Users were looking for a logical design, and some were left trying to find information through other routes (e.g. Google or the A-Z list of pages on the site) rather than finding what they needed intuitively.
(c) There was an opportunity to improve the accessibility of the site, so that it would be easier to use for the widest range of people, and to improve the experience of the site on mobile devices.
2. Additional satellite sites had emerged for different projects and events that needed to be incorporated.
3. The connection between the website and recording was not as strong as it could be,
meaning a key strategic goal of the Society was not being supported through the website.
It was recognised just how much high quality information was stored on the site, but it was spread across hundreds of pages and thousands of downloadable files, not doing the work it could! To balance the needs of specialist and expert users with those of beginners and the wider public, we needed to give due attention to full refresh. We also wanted to build a really strong foundation (in terms of the technologies used, the design and the editorial structure) for all our future web developments, and improve the environmental footprint of the website.
Aims of the project
From this starting point, we looked forward to consider what the website could, and should, be. Again, ideas coalesced quickly: a functional and attractive site should make it easy to find and access the right information quickly, no matter the knowledge of the user. It should present local and national resources seamlessly, making it easy to find out what is happening near yourself and across the organisation. And, last but not least, it should showcase the breadth, warmth and friendliness of the BSBI community.
Requested features included:
1. Better filtering options on content, such as by taxa, project, region or skill level.
2. Improved event and training listings, including for local (non-BSBI organised) events.
3. The continued provision of mapping.
4. More news presented in a better, more consistent and accessible manner.
The staff team also worked with the designer to review basic elements like readability, screen display and navigation, making decisions based on best practice.
The practical aspects
So, what have we done? As well working with an independent designer to achieve more practical
Working design of the new BSBI website home page
organisation of information, alongside updated look and feel, here are just a few of the practical steps the website redevelopment has taken so far:
• The underlying software of the website has been changed, to make it more secure, user-friendly and reliable.
• All content on the current site has been reviewed, updated, edited and migrated.
• A number of satellite sites, including the Governance site and New Year Plant Hunt site have been integrated into the main BSBI website.
• A new, far more functional members’ area has been added, with members using their own unique password, and being able to view information about their own membership, alongside member only content.
• County pages have been overhauled to standardise and better present the wealth of information on them. Vice-County Recorders will have the ability (following the initial roll out of the site) to suggest and create content for their pages, adding local events and making updates to content.
• Alternative language functions have been added, to make content more easy to display information in (for example) Welsh, where relevant.
• Site navigation has been overhauled, with a new organisational menu, search function, related content and linked pages.
Highlights
Some of the other exciting features we’ve been able to integrate into this first stage of refreshing the website, and that we hope will improve your experience, are:
• Integrating taxonomic information into the website, making it possible to link to taxa and search by genus and species directly for resources.
• Integration of our skills ladder into the design of the site, making it easier to find the content that is relevant to an individual no matter where they are on their botanical skills journey.
• Making better use of the fantastic plant imagery that has been contributed to the BSBI through the annual Photo Competition, through reports, and as part of the Plant Atlas 2020 project.
At the same time, we’ve integrated aspects of the current site that users told us were working well, such
The new BSBI website
as mapping, eBooks, contact details for individuals, and resources for recording, and maintained the wealth of information that was already available.
Timeline
We anticipate that, following the conclusion of migration, design and testing, the new site will go live this autumn. We will be working directly with those who look after information relevant to users, such as VCRs, committee members and local group teams, to walk through systems for getting information, including local events, onto the website going forwards.
Working through hundreds of pages and a small mountain of information has been a lengthy process, but we hope we can work together productively to make the site work for everyone.
We will be communicating with members about how to access the new members area – guidance and support will be provided – and about how to make the most of new features.
We are keen to hear from all users who might be interested in testing the new site, providing feedback on how it works, and what more it can achieve, over the coming months. Please do get in touch if you would be interested in taking part in testing by emailing enquiries@bsbi.org
Looking forward
We hope this first stage of overhauling the website will open a door to more ideas about how the site can work better for the largest number of people possible. The first phase of this development is intended to make our current website functionality and content more user friendly – but we are very much looking on how we can build on those foundations with further phases of development.
A number of opportunities for adding more information – e.g. bringing more species information onto the site – have already been identified for ‘Phase 2’. Once you have had a chance to see and use the new site, we would welcome any feedback or suggestions you might have. We will also be demonstrating the new website at this year’s British and Irish Botanical Conference.
Sarah Woods Fundraising and Engagement Manager sarah.woods@bsbi.org
NOTICES
ANNUAL GENERAL MEETING (AGM)
The BSBI Annual General Meeting 2025 will take place at 7.15 pm on Thursday 20 November 2025 as an electronic meeting. The AGM will be preceded by introductory talks starting at 6.15 pm. A registration link will be made available on the BSBI website for members who wish to attend. An invitation to attend the AGM is included inside this issue of BSBI News and posted on the BSBI website. The final agenda and all supporting papers will be also be available on the BSBI website which will be updated as the AGM approaches.
Any member interested in becoming a BSBI Trustee can find more information on the website (bsbi.org/bsbi-trustees).
If you wish to submit a resolution please contact the Honorary General Secretary by midday Thursday 6 November 2025. Submissions of proxy votes must be received by midnight Monday 17 November.
Barry O’Kane Honorary General Secretary hongensec@bsbi.org
Address for correspondence: c/o Julia Hanmer BSBI, 65 Sotheby Road, London N5 2UP
BRITISH AND IRISH BOTANICAL CONFERENCE 2025
This year’s British & Irish Botanical Conference will be held on Saturday 29 November at Edge Hill University, Ormskirk, Lancashire. The draft programme includes talks about BSBI research projects and publications, including Pete Stroh on the new GB Red List; talks about training, including Chantal Helm on FISCs and BSBI President Paul Ashton on teaching botany at Edge Hill. There will also be a panel discussion about botany and Artificial Intelligence; flash talks and exhibits from early career researchers; behind the scenes tours of the university’s scanning electron microscope; and Summerfield Books will have a pop up bookshop.
The draft programme and details of how to book for the event – whether you wish to offer an exhibit or you just want to come along and enjoy the day’s proceedings – can be found on the BSBI website: bsbi.org/british-irish-botanical-conference
The Conference is free to attend and to exhibit (although donations are always welcome) but please
do book in advance. We no longer provide a printed flyer or booking form for this event – very few people have used the form in recent years so we do not feel that the expense is justified. We are however very keen that members without internet access should be able to attend, so please contact Sarah Woods on +44 (0) 7570 254619 or at 23 Bank Parade, Otley, LS21 3DY, UK, to book or reserve an exhibitor space. We hope to record the talks and share them via our YouTube channel for anyone who can’t make it along on the day.
Louise Marsh Communications Officer louise.marsh@bsbi.org
NEW YEAR PLANT HUNT
BSBI’s fifteenth New Year Plant Hunt will run from 1st to 4th January 2026. Last year’s Hunt saw around 3,000 participants who submitted almost 25,000 records (a record number), noting more than 600 taxa in bloom. Our range of country level spotter sheets of the Top Ten and Top Twenty plants, alongside guidance notes, are available to help first time plant hunters.
Group hunts are always very popular and they are a great way for beginner botanists to get involved, so we’d love to see even more of them this time. If you feel able to host a group hunt – or if you’d like to hear how we can help you with organisation and promotion – please get in touch.
The data collected by our New Year Plant Hunters are helping us understand how our plants are responding to a changing climate, and the Hunt is a fun way for plant-lovers to get out recording in mid-winter. So please save the dates, register to take part and keep an eye on our webpage for further info over the coming months: bsbi.org/new-yearplant-hunt
Louise Marsh
WATCH OUT FOR SCAM EMAILS
We have recently heard from members who have been sent scam emails supposedly from the BSBI President. Scam emails often appear genuine but are actually fake. They might try and trick you to send money urgently, or reveal sensitive information. Always check the email address is genuine (see the website Who’s Who page or the Yearbook).
Please let us know about scam emails you receive, purporting to be from BSBI, by contacting me or enquiries@bsbi.org.
Julia Hanmer Chief Executive julia.hanmer@bsbi.org
STAFF CHANGES
Awarm welcome to three new staff who joined our team this summer and goodbye to another member of the team.
Northern Ireland Botanical Skills and Evidence Project
Jo Mulholland and Kim Lake have joined BSBI as part time Training Officers for this project, working to develop, deliver and facilitate a range of botanical skills training events and opportunities, and support the National Plant Monitoring Scheme (NPMS) in Northern Ireland. Jo has previously worked on community learning and education projects for RSPB and Ulster Wildlife Trust. She is a member of Glenarm Wildlife Group and volunteers for a whole range of monitoring schemes, including NPMS, bird ringing for the BTO and the National Moth Recording scheme. Jo is also involved in an RSPB project to restore Marsh Saxifrage in Northern Ireland. Kim started her career in IT, including as IT Manager for Alzheimer Society of Ireland, before changing to focus on ecology and botany, studying an environmental science degree and working on projects on woodland development, biodiversity action plans, bat surveys as well as volunteering as Secretary of BSBI’s Committee for Ireland.
Jen Farrar, Botanical Skills Officer NI, is leaving BSBI in September. We thank Jen for her service in the role. We are recruiting a new project manager and in the meantime, James Harding Morris, Countries Manager, will lead on project management of the Botanical Skills and Evidence project in Northern Ireland. We are grateful for support from the DAERA Environment Fund for this project.
Administration Officer
Jenna Poole joined BSBI in May, in the new role of Administration Officer, answering enquiries and assisting with events, engagement, membership, and other team administration. With a background in conservation, mixed with a fair amount of administrative and customer service, Jenna’s last job was with the Field Studies Council Biodiversity Team (who run the adult courses). She volunteered as a botanist in Roaringwater Bay in Ireland in 2007–
2009, and undertook some plant/habitat surveys in Lincolnshire in 2010–2012 and has been a BSBI member since 2010. Jenna is based in South Ayrshire. For staff contact details and a staff chart please see our Who’s Who page of the website: bsbi.org/ whos-who
Julia Hanmer
SKILLS AND TRAINING COMMITTEE VACANCIES
If you have an interest in botanical skills and training, BSBI’s Skills & Training Committee is looking for new members to help BSBI achieve its training-related strategic goals (bsbi.org/bsbistrategy). These include to deliver, encourage or support opportunities for learning, local engagement and participation for people of all botanical skill levels. The Committee meets twice a year. Its remit covers oversight of BSBI’s training provision, including FISC and Identiplant. Please contact me or visit the webpage to find out more: bsbi.org/volunteering-opportunity-bsbi-skillstraining-committee
Chantal Helm Training Coordinator chantal.helm@bsbi.org
BSBI AWARDS
In 2022, BSBI launched two new Awards to recognise those who go that extra mile to inspire and encourage their fellow botanists. The award for Outstanding Contribution to British and Irish Botany recognises a sustained and nationally significant contribution to advancing the understanding and appreciation of our flora. Individual BSBI members who make outstanding contributions to botany in their areas are recognised with our second award. Nominations can come from individual BSBI members, or from individuals on behalf of local groups or committees. For further details and to make a nomination see the BSBI webpage: bsbi.org/ nominations-awards
Apologies to Faith Anstey, the recipient of last year’s award for Outstanding Contribution to Botany in your Area, announced in the last issue. Due to an unfortunate mix-up, the photograph shown was of a different person.
Sandra (Sandy) Knapp Chair of the Board of Trustees chair@bsbi.org
AN UPDATE ON FISCS IN IRELAND
FISCs (Field Identification Skills Certificates) have been running for many years in the UK, but hitherto anyone from Ireland wishing to take this one day assessment of their botanical field skills has had to travel to England to do so – meaning that the cost and time involved can often be prohibitive, not to mention that Ireland has a quite different assemblage of habitats and plants. Therefore, to date very few Irish botanists have ever taken a FISC assessment.
So this year, for the first time, it was decided to trial a FISC assessment in Ireland, using plants sourced and collected from all corners of Ireland, to see how it would work in a practical sense and to gauge the level of interest and demand for such an assessment across the country. It is expected that FISCs will be especially popular with Irish consultants, who increasingly want to demonstrate evidence of their abilities, but they are also a really effective way of finding out where you sit on the botanical skills ladder and where your strengths and weaknesses lie.
For the uninitiated, the FISC assessment is composed of two time limited laboratory tests: ten common plants that must be identified without any resources, followed by twenty plants of varying levels of difficulty, from straightforward to fiendish, for which ID guides and keys may be used (but no electronic resources). These challenges are followed by a field test where candidates visit a previously unseen site of around 3 ha and record all the plants that they can find in two hours – again, as in real life, books and keys (but no phones) are allowed. Simultaneously, the site is examined by a previously appointed ‘Gold Standard Surveyor’, an accomplished botanist with (ideally) no prior knowledge of the site, against whose species list the candidates’ lists are marked. On the basis of the combined results of all three tests, candidates are assigned a grade ranging from 1 (beginner) to 5 (professional), with Level 6 awarded in exceptional circumstances.
The first ever FISC assessment to be held in Ireland was on Monday 21 July 2025 at Galway University campus, attended by eleven ‘guinea pigs’. Admittedly, this was only a trial, run at a greatly reduced fee, and no real certificates will be issued, but candidates got the chance to find out what a FISC in Ireland might feel like and were awarded informal grades to help them understand the process and identify how they might improve their skills.
The day was a great success, enjoyed by both the candidates and the organisers. Big thanks are due to the University of Galway, who very kindly provided the venue; all those who helped with behind the scenes organising, selecting and collecting specimens for the tests, assessing the trial candidates and undertaking the definitive survey against which candidates’ species lists will be compared; BSBI Training Coordinator Chantal Helm for setting the whole thing in motion and preparing for the day, and of course the ‘guineapigs’ themselves, who remained calm and cheerful in the face of even the most diabolical ID challenges and whose feedback will be invaluable in (hopefully) helping progress this into a regular event in Ireland.
Bridget Keehan Ireland Officer
bridget.keehan@bsbi.org
CHARLES GORDON HANSON (1938–2021) HERBARIUM SPECIMENS IN OLDHAM MUSEUM
Material collected and preserved by Gordon Hanson was recorded in his obituary (BSBI News 151, September 2022, 76–78) as being held in the following institutions: Natural History Museum, National Museum of Wales, University of Reading, and North Hertfordshire Museum. Missed from that list are those herbarium specimens at Gallery Oldham (formerly Oldham Museum) (OLDM). Gordon had been sending batches of unmounted specimens, wrapped in newspaper, to Oldham since 1999. He was first inspired to send specimens having read about Rev Charles Edward Shaw’s material already held at Oldham (BSBI News 79, September 1998, 36–39). One of Charles Shaw’s studies was the exotic plants of South Lancashire and West Yorkshire. He gathered specimens in 1950–1960s from wallpaper tips, council tips and sewage works.
The Gordon Hanson collection consists of about 800 specimens, now mounted on herbarium sheets, with collecting dates from 1976 to 2018. They are largely exotics with their origins in wool shoddy, soya bean waste, bird seed mixes, docksides and sewage works. Specimen origins are Somerset, Hampshire, Surrey, Bedfordshire, Hertfordshire and Norfolk. Most, however, are from Hertfordshire. As well as field material many Hertfordshire specimens were prepared from cultivated specimens from Gordon’s garden in Ware, the seed for these specimens being sourced both from the wild and from botanical
gardens across the world. Represented are the counties of Cornwall, Devon, Middlesex, Essex and West Yorkshire and the botanical gardens of Kew, Glasnevin and Edinburgh. Other countries represented are France, Italy, Sweden, Latvia, Hungary, Turkey, Czech Republic, Isreal, Uzbekistan, South Africa, China and USA.
Patricia Francis Natural History Curator, Gallery Oldham patricia.francis@oldham.gov.uk
REV G.E. SMITH (1804–1881)
Graeme Coles has produced an excellent little booklet (32 pages) on the life and discoveries of this Yorkshire clergyman, who made notable additions to the British native flora. He described three species new to science: Filago lutescens, Epipactis phyllanthes and Limonium binervosum, and was the first to find Eleocharis parvula new to Great Britain. He wrote a catalogue of south Kent plants (1829), with much on the orchids. He was instrumental in drawing J.E. Smith’s notice to the first known site of Orchis fuciflora outside Folkestone, which the latter published in his Vol. 4 of the English Flora (1828). Amongst other vice-counties, he also made important contributions to the botany of South Yorkshire and Derbyshire.
Graeme’s exemplary works on the discovery of the plants of South Yorkshire (2011) and Nottinghamshire (2023) are complimented by this really thorough little work.
Copies of the booklet, entitled A Cudweed New to Science, are available from Amazon for £5 – search for amazon.co.uk, click on books at the top of the page and enter ‘cudweed’ in the search box!
David Pearman dpearman4@gmail.com
BSBI HANDBOOK NO. 26: WILD ROSES OF GREAT BRITAIN AND IRELAND
The latest title in the series of BSBI Handbooks will be published this autumn, with a discounted pre publication offer available to BSBI members (see below and back cover of this issue for ordering details).
This new Handbook, authored by Roger Maskew and Gareth Knass, is the most comprehensive account of the wild roses of Great Britain and Ireland
ever published. It is the result of over thirty years field recording, as well as extensive literature and herbarium research, which has uncovered much previously unpublished historical information.
The Handbook is published in B5 format and covers 285 pages, of which over 200 are devoted to the species accounts that include descriptions and over 250 photographic illustrations of the 15 native species, over 20 aliens, and the majority of the 82 hybrids that have been recorded in the British Isles, many described and illustrated for the first time. Details of habitat, frequency and distribution, local variation, temporal changes and history are given for the majority of the species and more common hybrids.
A dichotomous key includes native species, the more frequent aliens, and three common and widespread hybrids. The detailed attention given to the descriptions and illustrations of the difficult and critical species and hybrids is a major feature of the species accounts.
Find out more about this new BSBI Handbook and view sample pages by visiting bsbi.org/bsbihandbook-wild-roses-of-great-britain-and-ireland Wild Roses of Great Britain and Ireland will retail at £25 but members can pre order this new BSBI Handbook now and pay only £16 / €18.50 (excl. P&P). Postage & packaging costs are: £3.50 (UK); £8.65 (Ireland and Europe), £14.00 (Rest of World) / €10 (Ireland and Europe), €16.60 (RoW).
There are three ways for you to pre-order your copy and claim your discount:
• Go to the password protected members’ area of the BSBI website where you will find simple instructions for placing your order, and a secure payment option. Email us at enquiries@bsbi.org if you’ve forgotten your password.
• Contact Sarah Woods by phone on +44 (0) 7570 254619 and place your order, or email her at sarah.woods@bsbi.org if you have any questions about payment.
• Send a cheque for the cost of the book and P&P to Gwynn Ellis (address on the inside front cover of this issue), including your full details.
A complimentary eBook of Wild Roses of Great Britain and Ireland will also be made available to you, along with your print copy, making it even easier to use this new title both at home and also in the field.
A standalone eBook will be published in January 2026 and will cost £14.
Sarah Woods Fundraising and Engagement Manager sarah.woods@bsbi.org
PURCHASING COPIES OF THE SPECIAL PLANTS OF EAST PERTHSHIRE
The review of this book in the Winter 2025 issue of BSBI News mistakenly stated that the publication was online as well as in hard copy. I would like to point out that the version online is an old (2019) version, which is much more basic, out of date, and really a totally different entity. The Special Plants of East Perthshire is only available from Summerfield Books and I commend it!
Martin Robinson
Vice-County Recorder for East Perthshire (v.c. 89) martin.robinson279@gmail.com
PANEL OF VCRS
There have been several new VCR appointments since April News. In England, Lionel Pike is the new VCR for South Devon (v.c. 3), taking over from Roger Smith. After a hiatus in Surrey without an official recorder since the retirement of Ann Sankey, v.c. 17 now has two new appointees: James Alvarez and Susan Medcalf. Richard Mabbutt has joined Alyson Freeman and Brian Laney as joint VCR for Northamptonshire (v.c. 32). In Wales, Many Forde has been appointed joint VCR with Nigel Brown for Anglesey (v.c. 52). In Scotland, John Gallacher is new in post for West Perthshire (v.c. 87) alongside Liz Lavery and Jane Jones, and there are no less than three new VCRs for Argyll & Kintyre (v.cc. 98 & 101): Ian Moir, Georgia Hancock and Jack Ravenscroft. In Ireland, Hannah Sheridan has been appointed joint VCR for West Donegal (v.c. H35), joining Ralph Sheppard, and Brendan O’Hanrahan is now joint VCR with Lisa Dowling in Co Carlow.
There are six retirements to report. As mentioned above, Pat and Dave Batty have passed the baton onto new VCRs after a tenure of 32 years in Kintyre. Three decades is an incredible length of time to volunteer for such a full workload in such a remote location, taking in two atlases along the way, so pick your own superlative for Ralph Forbes, who started as VCR for Fermanagh (v.c. H33) in 1977! Many of you will have marvelled at the Flora of the county written with co author Robert Northridge, but you may not know that fuller species accounts for almost 800 taxa were provided by Ralph and are available to download and read at bsbi.org/fermanagh. In Brecknockshire (v.c. 42), Mike Porter has retired after 13 years in post, Gordon Rothero has retired from Main Argyll (v.c. 98) after 8 years as VCR in this mountainous area,
and Pauline Hodson has stepped down as VCR for County Wicklow (v.c. H20) following a busy 12 years of recording alongside Catriona Brady. Pauline was also VCR for East Donegal (v.c. H34) from 1989–2013. The BSBI is nothing without such key volunteers who are the foundation for all the work that we do and the publications produced, and we thank them all very much for their time and service.
Pete Stroh Scientific Officer peter.stroh@bsbi.org
BRITISH & IRISH BOTANY 7:2
The latest issue of the 2025 volume of British & Irish Botany, BSBI’s open access, online scientific journal, was published in August. You can view or download all the papers free of charge, as well as previous issues and guidelines for submissions, from the B&IB website: britishandirishbotany.org/index. php/bib. You can also email or phone us on 07725 862 957 to discuss a proposal.
Stuart Desjardins & Louise Marsh bib@bsbi.org
British & Irish Botany Vol. 7 No. 2 (2025)
Past lessons and future requirements of reintroduction programmes for Cypripedium calceolus (Lady’s slipper Orchid, Orchidaceae) –David Trudgill
Symphytum × ferrariense (S. officinale × S. orientale, Boraginaceae) in Cambridgeshire, Cheshire and Norfolk: first records for England –Robert Leaney, Alan Leslie, Stewart Hinsley Coccoloba (Polygonaceae) achenes stranded on European Atlantic exposed shores – Dan Minchin, Declan Quigley, Edward Perry
Variation in the Ononis repens group (Fabaceae): implications for species determination and conservation – Christopher A. Skilbeck, Michael Wilcox
Summary of the current status of the montane and submontane plants of the Moffat Hills in Dumfriesshire – Christopher J. Miles
Three new British species of Hieracium (Asteraceae) – Brian Burrow, John Crossley, Tim Rich
Combinations in Pyrus (Rosaceae) required for inclusion in the second edition of Whitebeams, Rowans and Service Trees, BSBI Handbook No. 14 – Michael F. Fay, Maarten J. M. Christenhusz
BOTANICAL CROSSWORD NO. 35
by Cruciada
ACROSS
1. Travel with beers after jerk to get primordial trees (10)
8. Dog I give shout of approval to for finding leaf stem (7)
9. Photo that is covering journal (5)
10. Make verbal apology for fern feature (4)
11. Observed holding top ranking Assyrian plum (8)
13. Rice mixed in pail you’re said to end up with (5)
14. Endless broad leaved willow turned up in stewpots (5)
16. Fairy to fish for fleshy fruit foundation (8)
17. Flag up assistant for apple (4)
19. It goes round with scream of exhilaration from novice (5)
20. Old English stiff dismembered away from where it was originally found (3-4)
22. Tickle informer with cleavers (10)
Solution on p. 71
DOWN
1. Stares open mouthed as first root is taken from vine fruits (5)
2. Is 1 across an example of the inherent systems of the universe? (7,5)
3. Large organic constituent is forerunner for earth (3)
4. Poplars are useable when chopped up with crown removed (6)
5. Rio green transformed with fleabane (8)
6. I had crazy lot identifying orchid (12)
7. Parties of Fabaceae introducing zilch (6)
12. Fruit tree pest one can almost pick out in oddity (8)
13. Father heard to hesitate with tropical fruit (6)
15. Hangs low, you might say, with fleshy fruits (often stoned) (6)
18. Stalks and stops (5)
21. Fruit proverbially worthless? Returned present unopened (3)
COUNTRY ROUNDUPS
Compiled by Pete Stroh peter.stroh@bsbi.org
The Scotland roundup was delayed due to unforeseen circumstances and will appear in the next issue.
ENGLAND
The spring of 2025 was the driest in the UK for over a century, according to the Met Office. It was also the warmest and sunniest spring on record. As I write this note it is 31°C in Shrewsbury, my new stomping ground after moving from the ‘dry East’ at the beginning of the year. The upland grasslands have been tinder dry for months, and populations of spring ephemerals such as Moenchia erecta (Upright Chickweed), a species I particularly wanted to search out, were frazzled by late April. So much for the wetter, cooler west. The frequency of days over 30°C – which the Met Office terms ‘extreme heat events’ – have increased in England significantly in recent decades. Memories of exceptionally long spells of dry and warm weather, such as the drought of 1975/76, and more recently 2010–2012 and 2018–2019, are imprinted on many of us, but it is probable that in future climate scenarios, most especially in southern parts of England, such events will increasingly become the norm. One of the key questions for the BSBI is how our changing climate will affect the phenology of plants, and in turn the effects that altered flowering dates might have on invertebrate species and other wildlife. This year, a few volunteers have been trialling a survey methodology, modified by Kevin Walker from Preston (2020), which records the timing and abundance of all plants in flower along a fixed transect, with the route walked regularly throughout the year, ideally on a weekly basis. We hope that members will be interested in taking up this survey in 2026, and the Science Team will be publishing more about how to get involved in due course. There are numerous reasons which might lead to the discovery of a species in a new location. It is now almost a quarter of a century since Foot and Mouth disease led to the temporary cessation of sheep grazing in some areas that had been overgrazed for many years. This in turn allowed plants previously denied the opportunity to flower and fruit to do so, becoming more visible to the wandering botanist’s eye. One such species was
Carex vaginata (Sheathed Sedge), found by Rod Corner as a first English record on the Cross Fell range in Westmorland (v.c. 69) in 2002. Since then, various new sites for this sedge have been located in the northern Pennines, the most recent being this summer when it was recorded at an altitude of 720 m on Knock Fell by Tom Charman. The same lack of sheep grazing in 2002 produced a number of new records for Alopecurus magellanicus (Alpine Foxtail) and, coincidentally, another new site for this arcticmontane species was found this year, high (820 m) on Cross Fell, Cumberland (v.c. 70) by Anne Readshaw, David Freeman and others. Staying in Cumbria, there were particularly pleasing finds for two nationally threatened species. A splendidly vigorous specimen of Genista anglica (Petty Whin), covered in flowers, was found in Borrowdale by John Hooson and Chloe Lumsdon, and a field meeting of the Flora of Cumbria Recording Group in the wonderfully rich limestone area of Crosby Gill resulted in the refinding of a site for the lovely Bartsia alpina (Alpine Bartsia) where the plant had not been seen for more than 20 years.
Over in the north east, in South Northumberland (v.c. 67), Allium oleraceum (Field Garlic) has turned up at three subsites in muddy bays beside the River North Tyne, just upstream from the Tyne Watersmeet confluence near Acomb. It was previously thought extinct in the vice-county, although several historical sites beside the Tyne were previously known. An extensive population of another plant thought to be lost from S. Northumberland, Lepidium latifolium (Dittander), was found near Lynemouth last year, and has now been recorded in greater Newcastle,

Genista anglica (Petty Whin), Borrowdale (v.c. 70). John Hooson
and there are also second v.c. 67 locations for Carex pseudocyperus (Cyperus Sedge) close to where the Allium was found, and Epipactis phyllanthes (Greenflowered Helleborine) at Rothley Lakes.
The feeling one gets when finding a rare or unusual plant is one of the reasons why botanical recording can be such a pleasure. Not only is there the initial thrill of discovery, but also the awareness that associate species, perhaps some of them as uncommon as the initial find, might also be present. It is often not simply the individual plant in isolation that elicits excitement – context is everything to the discerning botanist. Three notable finds this summer included Oenanthe silaifolia (Narrowleaved Water-dropwort) in Buckinghamshire (v.c. 24), Sium latifolium (Greater Water-parsnip) in North-east Yorkshire (v.c. 62), and Hippocrepis comosa (Horseshoe Vetch) in Derbyshire (v.c. 57), found respectively by Andy McVeigh, Chris Bell and Chris Perry. These discoveries had two things in common. The first was that all are very rare in their respective vice counties; O. silaifolia is known from just two Bucks sites this century, S. latifolium hadn’t been recorded in v.c. 62 for 120 years, and H. comosa is the first record of the species in Derbyshire on magnesian limestone, the other few localities being present on carboniferous limestone. The second common factor was that further investigations led to the realisation that they had been intentionally introduced (although it’s just possible that the Hippocrepis is a natural occurrence, and not included in the seed mix sown), and without the knowledge of the VCR, which in turn led to the bursting of respective bubbles for the folk who reported the discoveries. It’s perfectly understandable that organisations or individuals would want to give threatened species a helping hand, and introduction can be a perfectly valid tool to assist recovery when all other options have been exhausted. However, at the very least the relevant VCR should be informed when an introduction does take place, and ideally a conversation would happen beforehand so that the full picture of its current and historical distribution is known, and receptor sites can be identified when appropriate.
As a model example of how a VCR has been actively involved in helping to attempt to reverse the fortunes of a threatened species, and documenting it meticulously, Stuart Hedley in Herefordshire (v.c. 36) has for some years been involved with the Critically Endangered Campanula patula (Spreading Bellflower). The aim of his work is to attempt to establish new populations where there is likely to be sustained disturbance and light provision
in the long term, whilst also monitoring the three extant native populations, all of which have very low numbers of plants. Crucially, Stuart will not introduce any plants to the extant native sites. By keeping the introductions separate, results of any future management work which might lead to natural recovery from the seed bank at the native localities will not be blurred by artificial ‘bolstering’ of the population. Indeed, only this year Stuart found two C. patula plants at a native site for which there were no records since 2000. The location, at Westhide, is on a bridleway that is kept open for public use, and so the species has benefited by unrelated (i.e. not conservation-led) operations. The previous spot where the bellflower was known, c.30 metres away, is now an overgrown coppice and no longer suitable. Stuart’s methodology, clearly thought through, seems an entirely acceptable course of action for a plant which was once widespread in Herefordshire. Three introductions are planned, and one of these has already had a pilot transplant – the other two will be finalised before next autumn.

Travelling down to the Channel Islands, approximately 120 spikes of Epipactis helleborine (Broad leaved Helleborine) were chanced upon in a small woodland on Jersey by Tom Crace (det. Tim Rich) whilst venturing into the wood during a break from strimming roadside margins. This
Campanula patula (Spreading Bellflower) at an introduction site in Herefordshire (v.c. 36).
Stewart Hedley
appears to be a first for the Islands, discounting three dubious historical records from Guernsey on the BSBI database. The population must have been hiding for some considerable time, given its size. On the Isles of Scilly, a well-known species to the islands, and potentially a highly invasive one, Cotula coronopifolia (Buttonweed), drew attention to a surprising near neighbour. The non native was found by Dave Mawer growing in a shallow coastal pool on White Island, an uninhabited place off St Martin’s (see p. 25). Attempts were made to pull it up, and photographs taken and sent to Rosemary Parslow. When Rosemary looked closely at the photo, she realised that the plant growing with the Buttonweed was Suaeda maritima (Annual Sea blite). This is only the second record for Scilly, with the previous record from Bryher 20 years ago. Access to remote islands can prove difficult, as can recording on private land.
A landscape recovery project, ‘Small is Beautiful’, began this year on Scilly, with surveys led by Ian Bennallick and Liz Askins taking place on all participating arable farms. It was a fantastic opportunity to visit areas usually off limits to botanists and see how different management impacted on arable weeds. Amongst the target species recorded were Silene gallica (Smallflowered Catchfly), Chrysanthemum segetum (Corn Marigold), Stachys arvensis (Field Woundwort), Lotus subbiflorus (Hairy Bird’s foot trefoil), Briza minor (Lesser Quaking-grass), Ranunculus parviflorus (Small flowered Buttercup), R. muricatus (Roughfruited or Scilly Buttercup) and Fumaria occidentalis (Western Ramping fumitory). Not a bad haul! As an added bonus, a new site was found for Lythrum hyssopifolium (Grass-poly) growing in field ruts.
A plant that sadly appears to have been lost from Scilly, Rumex rupestris (Shore Dock), has happily been re found by Rob Large at a South Devon (v.c. 3) location where it had not been seen for over 20 years. At least 10 plants were confirmed near Gara Rock, west of Prawle, alongside the much more common R. crispus subsp. littoreus and possible hybrids.
Sticking with coastal discoveries, while attending a joint meeting of the local BSBI Cheshire recording group with the Wirral Recording group and the Cheshire Wildlife Trust at Red Rock Sands in July, Sam Thomas found Calystegia soldanella (Sea Bindweed) growing on a recently accreted sandy bank. This represents the first Cheshire (v.c. 58) record of the species since the 19th century. In North Devon (v.c. 4) Galium Referee Alex Prendergast, while visiting Braunton Burrows NNR, identified a population of Galium constrictum (Slender Marsh

Bedstraw) in a dune slack, new for the vice-county. In Devon, it has only ever been known from the south (v.c. 3), where it occurred in the Chudleigh Knighton area but was lost by the late 1960s (Smith et al., 2016). Remarkably, another new site for this Bedstraw was found in Shropshire at Cole Mere this year by Kat Newbert (det. Alex Prendergast), very far from its known principal GB distribution in the New Forest. Some time ago the Plant Crib (Rich & Jermy 1998) suggested that G. constrictum might be overlooked away from its known haunts, and over a quarter of a century later these records would seem to corroborate this view.
Himantoglossum hircinum (Lizard Orchid), unlike G. constrictum, is hard to overlook when in flower and has been spreading throughout southern England for the past two decades. In 2025, a single plant was recorded by Ged Keele in flower on Brent Knoll, North Somerset (v.c. 6), representing the first new site in Somerset since 1978, and the most western in GB. It has also appeared in the grassland of a private garden in Warwickshire (v.c. 38), a first record for the vice-county.
Some plants have a habit of jumping the garden fence, making the assignation of a native or alien status very difficult to pin down. In
Rumex rupestris (Shore Dock), Gara Rock, near Prawle (v.c. 3). Rob Large
Shropshire (v.c. 40), two subpopulations of Allium scorodoprasum (Sand Leek) were found almost simultaneously by two botanists, John Martin and Rob Rowe, who were coincidentally both recording at Clun Castle (see photo on front cover of this issue). The plants were growing in rather scruffy Arrhenatherum elatius-dominated grassland on sandy soils, not unlike the habitat at its more northerly native locations. Shropshire sits in the noman’s land between the native and alien mapping of its distribution, and this find represents a new vice county record. No plants were seen in cultivation nearby.
Red or blue? Sometimes it’s just nice to know a plant is present, and celebrate the discovery. A plant that would have been red inland in the New Atlas, but blue in Plant Atlas 2020, is Triglochin maritima (Sea Arrowgrass). This halophyte, unlike other more familiar examples, is very rarely found away from the coast and so its discovery by John Hodgson, along Ringinglow roadside by Lady Canning’s Plantation, is notable and a first record for land locked Derbyshire. No matter where you live in England, the sight of Lysimachia tenella (Bog Pimpernel) is always a welcome one and a sign of good quality vegetation worth exploring. Near to my new home it is a frequent component of upland flushes, but across much of the lowlands of England it is increasingly restricted to protected areas. It has long been a rarity in South Lincolnshire (v.c. 53), last seen in 1992 until this year, when Sarah Lambert and Pete Kirby discovered a population in a groundwater-fed pool at Dunston, near Lincoln. The same week, Kerry Harrison made an excellent find at Baston Fen Nature Reserve; a population of Sonchus palustris (Marsh Sow thistle) comprising 17 flowering spikes within the reserve and four additional plants on a nearby bank. This Nationally Scarce species has not previously been recorded in v.c. 53, though there is an extant population at South Ferriby in North Lincolnshire (v.c. 54). It has been confirmed that this species has not been deliberately introduced, so the most plausible explanation is natural colonisation from established populations in Huntingdonshire or Northamptonshire.
In nearby Cambridgeshire (v.c. 29), the number of sites for Orchis anthropophora (Man Orchid) was doubled in 2024 after its discovery by Lucy Watts at Fulbourn Fen, and as with buses, another new location has been found this year, this time by Iain Webb, who spotted a flowering spike while cycling along a candidate protected road verge at Babraham. This represents a rather remarkable upturn in the fortunes of a species locally that has
experienced decline across much of its national range in recent decades. Two arable plants that are also now threatened in GB have been recorded in new locations. Lysimachia foemina (Blue Pimpernel) was spotted by Lucy Wilson (and later confirmed microscopically) on a species rich arable margin in Madingley, having last been recorded in Cambridgeshire in 2013, and Valerianella dentata (Narrow fruited Cornsalad) was identified during a meeting of the Cambridgeshire Flora Group at Dungate Farm. A new population of another scarce species that appreciates regular disturbance of the soil, Filago pyramidata (Broad-leaved Cudweed), has been confirmed for North Hampshire (v.c. 12) by Tim Rich following its discovery by Tristan Norton at Long Valley near Farnborough, a military site used to test vehicles. The site is essentially wooded heath with extensive areas of churned, damp sand used as heavy vehicle race tracks. In recent years some nice plants have been found there, including Lythrum hyssopifolia (Grass-poly) and a colony of Pyrola rotundifolia (Round-leaved Wintergreen).
In South Hampshire (v.c. 11), Hypopitys monotropa (Yellow Bird’s nest) was spotted by Frankie Copsey at Ampfield Woods just prior to her taking part in a cross country race. This unobtrusive plant was last seen at this location in 1936, and has records from just 9 monads in the vice-county this century. It is an even rarer species in North Devon, with the find of 86 plants on Braunton Burrows by Mary Breed representing only the second location for it in v.c. 4. Indeed, it may be the sole survivor, as it now appears to have been lost from its other site at Old Yelland Power Station. There is also a new hectad record for the species in Surrey (v.c. 17), where it is much more frequent relatively, but with very few records to subspecies. Jane Lowe determined the plants she found at Selsdon Wood as subsp. monotropa, and this was subsequently confirmed by Fred Rumsey (see Fred’s article in the last issue for a useful Hypopitys key). This is the first vice county record since 1958! Susan Medcalf, joint VCR for Surrey, has also sent news about many other notable finds for the county this year, including new hectad records for Chamaemelum nobile (Chamomile) on Elstead Cricket Field, a new site for Ophrys insectifera (Fly Orchid) at Hackhurst Downs, and Pyrola rotundifolia subsp. rotundifolia (Round-leaved Wintergreen), found by George Hounsome at Bisley Common in 2023 – a bit late to the party in terms of publication, but rectified now. The latter is only the second site in v.c. 17.
Quite a few of the records above have been discovered in well botanised places, demonstrating
COUNTRY ROUNDUPS: England / Wales
that there is always more to detect even on welltrodden turf, and so it was at Cothill Fen, just over the border from Oxfordshire (v.c. 23) into Berkshire (v.c. 22), when David Morris spotted Blysmus compressus (Flat sedge), a new site for the vice county with very few known locations historically, and only one other surviving into the 21st century. This lovely sedge was also found at a new site in East Cornwall (v.c. 2), Cubert Common, 2 km north of an extensive population at Penhale Sands. In East Sussex (v.c. 12), there is a new site for Dactylorhiza incarnata (Early Marsh orchid) at Ashdown Forest, and more spectacularly, a first East Sussex (v.c. 14) record since the 1860s for Cirsium eriophorum (Woolly Thistle) by Graham Lyons at Rottingdean. Some species can be hard to spot, even when you are standing right next to them, and I count Parapholis strigosa (Hard Grass) as one of these, especially on overgrazed saltmarsh. Dave Barlow has let me know of a fifth site for this grass in North east Yorkshire (v.c. 62), and also a fourth post-1999 location for Carex muricata subsp. pairae (Smallfruited Prickly sedge), found by Alistair Fitter.
Taxa in the C muricata group can be fiendishly tricky to identify – on a par with Lemna (Duckweeds), perhaps? Look at the L. gibba (Fat Duckweed) BSBI distribution map and you will see a big blank space in the south west. That’s no longer the case after Dave Steere found fronds in trackside puddles near Week St Mary (v.c. 2), later confirmed by Richard Lansdown. Richard also confirmed the find of Lemna valdiviana (Valdivia Duckweed), made by Steve Woodward and Rob Smith at a new pond in Sheepy Magna, Leicestershire (v.c. 55), presumably arriving via plants bought from a garden centre. This is a first record for the vice county, but almost certainly not the last!
References
Preston, C.D. 2020. The phenology of an urban street flora: a transect study. British & Irish Botany 2: 1–26.
Rich, T.C.G. & Jermy, A.C. 1998. Plant Crib 1998 Botanical Society of the British Isles, London. Smith, R.E.N., Hodgson, B. & Ison, J. 2016. A New Flora of Devon. The Devonshire Association for the Advancement of Science, Literature and the Arts. Exeter.
Pete Stroh Scientific Officer
WALES
We’ve just had an excellent few days at the Wales Annual Summer Meeting and AGM at Rhyd y Creuau in Denbighshire (v.c. 50), where we were treated to an array of excellent field visits, organised brilliantly by Delyth Williams and co. On a visit to Pen yr Orsedd bog near Pentrefoelas, in addition to the known about niceties like Carex limosa (Bog Sedge), Wahlenbergia hederacea (Ivy leaved Bellflower) and Rhynchospora alba (White Beak-sedge), Lindsay-Anne Heald also found Osmunda regalis (Royal Fern) which was new to the hectad and one of very few extant sites in v.c. 50. The group also had visits to Bryn Euryn SSSI where they saw Helianthemum oelandicum (Hoary Rock-rose), Astragalus glycyphyllos (Wild Liquorice) and Rosa agrestis (Small leaved Sweet briar); Pensarn Beach with its Glaucium flavum (Yellow Horned-poppy), Medicago sativa nothosubsp. varia (Sand Lucerne) and Crambe maritima (Sea Kale); as well as to Capel Garmon, Bodnant Gardens and to the RSPB reserve at Conwy where the group made nearly 500 records on the day, entered by Jonathan Shanklin on the BSBI Recording App. We also had a talk by Tim Rich about Stachys alpina (Limestone Woundwort) about why the species should be considered a native.
There have been some good refinds made as part of the BSBIs project on Sites of Special Scientific Interest (SSSIs) in Wales, including Convallaria majalis (Lily of the Valley) found by Stephen Marshall on Mynydd Du SSSI in Breconshire (v.c. 42), a first record for about 35 years, Salix herbacea (Dwarf Willow) on Arenig Fach in Merionethshire (v.c. 48), a first hectad record for 50 years, Dactylorhiza purpurella var. cambrensis (a Northern Marsh Orchid) on Glaslyn SSSI in v.c. 48 (a first here for over 30 years) and Oenanthe fistulosa (Tubular Water Dropwort) re found by Arthur Chater, Julian Woodman and Tom Kistruck at its only Merionethshire locality.
Also in v.c. 48, Euphrasia cambrica (Welsh Eyebright) was re found on Cadair Idris by the BSBI field meeting group that spent two back to back days in July scouring its former locations (last recorded here nearly 40 years ago). In addition to E. cambrica, the mountain also produced E. frigida (Upland Eyebright), a new species to Wales, which was in a base rich flushed rocky gully below one of the Saussurea alpina (Alpine Saw-wort) locations. I say ‘one of’ because 2025 has also seen a new monad record for S. alpina on Cadair Idris by Matt Sutton, along with Trollius europaeus (Globeflower) high on the mountain. The group also saw other Cadair Idris specialities including Genista pilosa
(Hairy Greenweed) which wasn’t flowering here, although it was seen flowering in Breconshire this year, spotted by Christeen Grant. Nobody seems to know if it has ever been seen flowering before at the Breconshire site, but certainly not recently. There was also more of it there than previously thought, as it hides under vegetation very well.

Genista pilosa (Hairy Greenweed), Breconshire. John Crellin
In south-west Wales, the project also rerecorded Lathyrus palustris (Marsh Pea) at its only Pembrokeshire (v.c. 45) locality on marshes by the Cleddau Ddu, and then the following day Steve Chambers, Yusef Samari and Alastair Hotchkiss found L. palustris on the Teifi Marshes in Cardiganshire (v.c. 46) – a new species to the vice county. The only reason that the group were battling through dense swamp vegetation was in the hope of re finding Carex punctata (Dotted Sedge) which wasn’t relocated, but the pea more than made up for this!
The start of 2025 has also seen another new-toCardiganshire species, with Allium ampeloprasum (Wild Leek) being found by Steve Chambers on Constitution Hill just north of Aberystwyth in April 2025. A total of half a dozen or so plants occurred in three separate places distributed across three different monads. They must have been present for some time as Steve later found out that Mary Moylett had photographed two of them while walking her dog along the same track in 2023/24. They are currently under observation to see which of the two bulbiferous varieties they are assignable to.
Another new-to-v.c. in south-west Wales was in Carmarthenshire (v.c. 44), where Laura Moss discovered a single large plant of Hypericum hirsutum (Hairy St. John’s wort) just coming into flower during a West Wales Biodiversity Information Centre recording day to a farm near St. Clears in
COUNTRY ROUNDUPS: Wales
June. Apart from a single record in Pembrokeshire, this is the only record of H. hirsutum in south-west Wales. It was growing in a small area of tall herb vegetation by a farm building close to the site where Matt Sutton found nine Dianthus armeria plants in 2021. Over fifty D. armeria were seen on the recording day.
Elsewhere in Carmarthenshire, Kath and Richard Pryce re-found Gymnocarpium robertianum (Limestone Fern) at Carreg yr Ogof on Mynydd Du, at its only site in v.c. 44. First recorded in 1907 by H.H. Knight, it was last noted by Ian Morgan and the late Nigel Stringer in 2012 when they recorded sixteen plants. Kath and Richard’s visit also resulted in finding Botrychium lunaria (Moonwort) new to the hectad.
In other vice counties, Monmouthshire’s (v.c. 35) highlights from the first part of 2025 included a new site for Anacamptis morio (Green-Winged Orchid) during meadow surveys by Steph Tyler with Wendy Tyler-Batt, and new sites for Parapholis incurva (Curved Hard-grass) on the Caldicot Levels east of Newport recorded by Elsa and Adrian Wood; plants of Rumex cristatus (Greek Dock) at Rogiet below the sea wall and Goldcliff on rocks below the sea wall; as well as new records of Ceratochloa cathartica (Rescue Brome) from Caldicot Moor and Pill Farm on the Levels. Lowri Watkins also reported 21 plants of Orobanche rapum-genistae (Greater Broomrape) at Springdale Reserve near Usk.
On Anglesey (v.c. 52), the local flora group have been on many outings in the first part of 2025, with lots of nice finds including Dactylorhiza traunsteinerioides (Narrow-leaved Marsh Orchid) at Waun Eurad. There have also been efforts this spring to re-record Mibora minima (Early Sand-grass) in some detail by the Anglesey Flora Group and Natural Resources Wales. Elsewhere John Bratton re-found Hypopitys monotropa (Yellow Bird’s nest) near Menai Bridge.
In Montgomeryshire (v.c. 47), Kate Thorne and Fiona Gomersall refound Sorbus torminalis (Wild Service-tree) at Coed Ty Mawr SSSI in May as well as Carex strigosa (Thin-spiked Wood-wedge), which was a new hectad record. A new site was found in June for Alopecurus aequalis (Orange Foxtail) by Richard Bullock in the floodplain of the River Vyrnwy. Other significant new finds include a second v.c. 47 record of Setaria pumila (Yellow Bristle Grass) by Fiona Gomersall at Dolydd Hafren and a concerning first vice county record of Hydrocotyle ranunculoides (Floating Pennywort) which came in via iRecord. Other aliens making their way into the county include Polypogon viridis (Water Bent) recorded in Welshpool at its second v.c. 47 locality. This species
has also now made its way to v.c. 48, with records coming in 2025 from Dolgellau and Barmouth. Also in Barmouth (v.c. 48), Malva neglecta (Dwarf Mallow) was re recorded, and in Aberdyfi it was found this year by Heather Garrett, updating an unpublished record from 1969 by Peter Benoit.
Alastair Hotchkiss
Wales Officer
IRELAND
The spring of 2025 was a lovely one: sunny, calm and maybe even a bit too dry: quite an unusual combination of conditions here in Ireland. Everything got off to a flying start, and continued to flourish through a warmer-than-usual summer, with the net result that at the time of writing, I’m left feeling slightly dazed, with a sense of much having passed by in a flurry.
It has been a great summer for refinds and new discoveries from our intrepid team of recorders. In addition, there has been a fantastic programme of field events, including eight Targeted Aquatic Plants Project (TAPP) outings, which this year have focused on refinding or revisiting some rare aquatic species at key sites, thanks to funding from NPWS.
A TAPP trip to Co Kerry (v.cc. H1 & H2), led by Paul Green, found Subularia aquatica (Awlwort) at Cummeenduff Lough (two plants, where it hadn’t been seen since 2002), and at Lough Duff, where it was found abundantly after a 50 year gap. Gratifyingly, Wahlenbergia hederacea (Ivy-leaved Bellflower) was also abundant along the banks of Cummeenduff Lough, while Nitella translucens (Translucent Stonewort) was found on the west side of Lough Duff, only the second record for the hectad.
Sadly, the area at Lough Gealain, Co Clare (v.c. H9), where thousands of Nitella tenuissima (Dwarf Stonewort) were seen last year, had dried out during spring; although the water was deep again by late May, it was evidently too late for it to grow. However, the Lough proved great for pondweeds: led by Nick Stewart and Paul Green, the group found Potamogeton × angustifolius (P. lucens × gramineus) – but neither of its parents – and also P. coloratus (Fen Pondweed), P. praelongus (Long-stalked Pondweed) and P. natans (Broad-leaved Pondweed). The following day, in the River Fergus at Ennis, lots of P. × salicifolius (P. lucens × perfoliatus, Willow-leaved Pondweed) was found, first seen here in 1972 by N.D. Simpson. Yet only one of its parents, P. perfoliatus (Perfoliate Pondweed) was observed, along with P. crispus (Curled Pondweed) which proved to be a
new hectad record. A good day for hybrids: Rumex × weberi (Broad-leaved Dock × Water Dock) and Veronica × lackschewitzii (Blue Water-speedwell × Pink Water speedwell), both new for the hectad, were found on the bank of the river.
A July visit to South Slobs in Co Wexford (v.c. H12) found Ceratophyllum submersum (Soft Hornwort) – listed under the Flora Protection Order (FPO) in Ireland – which was abundant in the many drains examined. In a black, sludgy drain with very little water, the group updated the hectad’s only site for Ruppia maritima (Beaked Tasselweed) – its fruits indicating var. brevirostris. A bare gravelly area by the pumphouse provided a new site for Puccinellia fasciculata (Borrer’s Saltmarsh grass), also a FPO species, growing with P. distans (Reflexed Saltmarsh grass).
The following day, at Tacumshin Lake, Lamprothamnium papulosum (Foxtail Stonewort) and Chara canescens (Bearded Stonewort), were recorded by the thousands, growing amongst Bolboschoenus maritimus (Sea Club rush) on the lake margin. Ruppia maritima (Beaked Tasselweed) was very abundant (this time var. maritima), growing with R. spiralis (Spiral Tasselweed), Zannichellia palustris subsp. pedicellata (Horned Pondweed) and Stuckenia pectinata (Fennel Pondweed).
Paul revisited Tacumshin lake on 24 July, where he recorded Lemna turionifera (Red Duckweed) – a new duckweed for Ireland – growing in a large pool (formerly part of Tacumshin Lake, before this was invaded by a large reedbed) with L. minor (Common Duckweed), L. minuta (Least Duckweed), L. gibba (Fat Duckweed), L. valdiviana (Valdivia Duckweed) and Spirodela polyrhiza (Greater Duckweed), as well as Potamogeton berchtoldii (Small Pondweed). Paul observed that as L. turionifera looks rather similar to Lemna gibba, it’s quite likely to be overlooked. It is red-coloured around the junction of the root and the frond, and has a row of bumps along the centre of the upper side of the frond, visible to the naked eye once you know they are there! Its rather remote location suggests it was introduced by birds. A nearby pool, not part of Tacumshin Lake, revealed a new population of Ceratophyllum submersum: for such a rare species, it certainly keeps turning up in new sites!
Hannah Mulcahy led a field meeting to Carran Turlough, Co Clare, on 21 June. Exceptional weather made for a lovely day of recording, taking in the views while exploring the turlough basin and areas of limestone pavement. The highlights were Orobanche alba (Thyme Broomrape), Adiantum capillus-veneris (Maidenhair Fern), Saxifraga rosacea
(Irish Saxifrage), Gymnadenia conopsea (Fragrant Orchid), Dactylorhiza incarnata (Early Marshorchid), Platanthera bifolia (Lesser Butterfly orchid), Ophioglossum vulgatum (Adder’s tongue) and Cerastium arvense (Field Mouse ear).
Also in H9, Micheline Sheehy Skeffington was surprised that her discovery of Stratiotes aloides (Water-soldier) at Gweeny Churchyard was a new record for the county, as she knows of a few locations for this species in nearby SE Galway. Drymochloa sylvatica (Wood Fescue), recorded by Micheline with Cilian Roden on the edge of the Slieve Aughty Mountains, also proved to be a new record, not just for the county but the whole Clare/Galway region: Micheline mentioned that, although recorded there by Praeger, this is not noted in the DDb. This shallow rooted grass grows on rocky substrates, often in inaccessible locations, so this is a truly impressive find.
In Co Galway (v.cc. H16 & H17), Ciarán Bruton was delighted to record Calamagrostis epigejos (Wood Small reed) north of Moycullen, the first ‘mainland’ record from West Galway, while Euphorbia paralias (Sea Spurge), new to Galway, turned up at Inverin: Phoebe O’Brien originally found this population, not flowering at the time, in 2020. Recording for BSBI’s Irish Upland Plants Project – which aims to refind occurrences of a target list of upland species that have not been seen this century – Ciarán found Huperzia selago (Fir Clubmoss) in a blanket bog northwest of Galway City: a first for the hectad since the 1960s. Galway City turned up several new aliens: Melissa officinalis (Balm), Diplotaxis tenuifolia (Perennial Wall-rocket), Mercurialis annua (Annual Mercury) and Papaver somniferum (Opium Poppy), as well as a seemingly long-persisting population of Lathyrus latifolius (Broad-leaved Everlasting-pea). Browsing the Phoenix canariensis palms in the Galway B&Q, Ciarán noticed a couple of hitchhikers: Melilotus sulcatus (Furrowed Melilot) and Galinsoga parviflora (Gallant Soldier), neither recorded in the west of Ireland previously. He believes these plants were imported from Italy. Meanwhile, completing the regiment, Chris Peppiatt found G. quadriradiata (Shaggy Soldier) in Galway City in July, as well as Melilotus officinalis (Ribbed Melilot) at Lough Atalia in June: both new species for H17 (North east Galway).
In his role as VCR for Longford (v.c. H24), Ciarán Bruton led a field meeting to the cutaway peatland at Corlea Bog, and was able to show off Pyrola rotundifolia subsp. rotundifolia (Round-leaved Wintergreen), a new species for the County which is very sparsely distributed across Ireland.
COUNTRY ROUNDUPS: Ireland
In Sligo (v.c.H28), I and my friends Annette Williamson and Justin Lyons from Ceredigion were delighted to see the Benbulben speciality Arenaria ciliata (Fringed Sandwort), looking absolutely stunning and surprisingly abundant on a warm sunny day in mid-May. The same week, we found Rubus saxatilis (Stone Bramble) at Carrowmore above Geevagh, not recorded in the hectad since 1998 –one of the target species highlighted in Ireland’s new Upland Plant Project. In early July, Eamon Gaughan and I were delighted to discover a new site for Trocdaris verticillata (Whorled Caraway) in a damp meadow near Grange, only the second site for the county. As always, Eamon has recorded prolifically in both Sligo and Leitrim (v.c. H29) this summer and has discovered several new taxa for the region, including Hordeum murinum (Wall Barley) at Finisklin and the neophyte Oxalis incarnata (Pale Pink-sorrel) at Slieveroe, Co Sligo.
In May, Helen Carleton found a horsetail resembling Equisetum variegatum (Variegated Horsetail) in the new dune system at Tramore near Rosbeg in West Donegal (v.c. H35). However, it appeared significantly more robust than usual, so in July Robert and Hannah Northridge revisited Helen’s patch (covering about 10 m × 10 m), and sent some stems to the BSBI referee Fred Rumsey. Fred concluded that it is indeed a large form of Equisetum variegatum, but warns that horsetails can be particularly variable in Ireland – something to bear in mind when horsetail hunting! Nonetheless, this is an excellent find for H35, representing a new hectad record.
Helen has also been participating in the Upland Plants Project and recently re-recorded Empetrum nigrum (Crowberry), Dryopteris aemula (Hay-scented Buckler-fern) and Solidago virgaurea (Goldenrod), as well as numerous Huperzia selago (Fir Clubmoss), on the Leitrim side of the Playbank, near Ballinagleragh, none of which had been recorded in this monad since 1998. She also found numerous locations for Hymenophyllum wilsonii (Wilson’s Filmy fern): the first ever records for the Leitrim side of hectad H02.
In Antrim (v.c. H39), in late July, David McNeill recorded Sorbus rupicola (Rock Whitebeam) from Garron Point (last seen in 2008) and Hypopitys monotropa (Yellow Bird’s nest) from Straidkilly, not recorded there since 2011. His discovery of Veronica polita (Grey Field speedwell) at Killycarn in May proved to be a new vice county record for this neophyte species, more typically found in the south and east of the country, favouring arable margins and waste ground.
COUNTRY ROUNDUPS: Ireland
Inspired by the Upland Plants Project, David visited Trostan in H39, finding two populations of Lycopodium clavatum (Stag’s horn Clubmoss) and a new hectad record for Botrychium lunaria (Moonwort). Another upland excursion to Mullaghmore in H40 (Derry/Londonderry) turned up a very healthy population of Salix herbacea (Dwarf Willow), not recorded in the hectad since 2013, plus two new sites for Botrychium lunaria and a new species for the County, Epilobium pedunculare (Rockery Willowherb)! This New Zealand native was not recorded growing wild in Ireland until 1953 and, although spreading, remains most frequent in the mid-west.
In Co. Down (v.c. H38), Graham Day recorded some exciting new finds this year: Malva neglecta (Dwarf Mallow) by Loughkeelan, the first record since 1915; Geranium pusillum (Small flowered Crane’s bill) at Ballywalter, the first county record since 1960; and the first Irish record of Salvia × jamensis, found in a hedge at Killowen.
John Faulkner has been busy writing up his forthcoming Flora of Co Armagh (v.c. H37) so has had less time than usual for recording, but nonetheless found a new site for Spiranthes romanzoffiana (Irish Lady’s tresses) on a peaty meadow at Derryhubbert, just south of Peatlands Park. There was just one

single spike, in perfect condition: excitingly, this is the first record for the vice county since 1986.
In Waterford (v.c. H38), Ann Trimble and Andrew Malcolm were delighted to count 66 fronds of Trichomanes speciosum (Killarney Fern) growing beside the River Owenashad, close to where they live. Ann found a single Epipactis helleborine (Broadleaved Helleborine) at the new site of Camphire by the river Bride in Co. Waterford.
Even going to work can turn up new things: in March, Ann found Urtica membranacea (Mediterranean Nettle) in full bloom near her office in Clonmel, South Tipperary (v.c. H7). A new species for Ireland, this first appeared in the UK in 2006 and has occurred there sporadically since, but being frost sensitive, doesn’t tend to persist. Plant Atlas 2020 suggests it may arrive with imported horticultural plants, or on vehicles coming from southern Europe, so can we expect more records, and will it manage to persist here given our milder, damper climate?

Another interesting Clonmel find for Ann was a white variant of Ivy Broomrape – Orobanche hederae f. monochroa, which has only been noted in five other locations in Ireland.
Paul Green has again done an amazing amount of recording, with some wonderful new finds for Wexford (v.c. H12). Eleven plants of the FPO species
Spiranthes romanzoffiana (Irish Lady’s-tresses), Derryhubbert, Co Armagh (v.c. H37). John Faulkner
Urtica membranacea (Mediterranean Nettle), Clonmel (v.c. H7). Ann Trimble
Trifolium glomeratum (Clustered Clover) showed up on a steep road bank, growing with Lotus subbiflorus (Hairy Bird’s foot trefoil) and Trifolium striatum (Knotted Clover) – also exciting finds! – during a field meeting to Upper Ballyconnigar in late May. This is a new area for the species, 18 km from the closest known site at Rosslare, where it was first recorded by E.S. Marshall in 1897. Wexford remains its stronghold, with a third thriving colony at Ballyhack; unseen since the 1920s at its only Wicklow site, and not recorded at its single Waterford site since 2005, it is unknown from the rest of Ireland.
A dried-out pond at Kilmichael, in north H12, revealed another FPO species new for the vice county: Alopecurus aequalis (Orange Foxtail) – also recorded this year by Hannah Mulcahy at a known turlough site in south Limerick (H8). This species favours bare, muddy places that are wet in winter but dry in summer, such as pond and ditch margins, so
OBITUARY NOTES
Sincewe compiled the last Obituary Notes, news has reached us of the death of the following members, some of very long standing. We send our sympathy to their families and friends.
Mr P.J. Corbett of Awsworth, Nottinghamshire, a member for 30 years. Mr M.J. Degg of Kingswinford, West Midlands, a member for 37 years. Mr M. Porter of Crickhowell, a member for 56 years and former VCR for Breconshire. Dr C. Skilbeck of Sittingbourne, a member for 9 years. Mr A.V. Smith of Stockport, Cheshire, a member for 57 years. Mr D.J. Tennant of Kilnsey, Yorkshire,
the recent dry weather has probably helped it along – though at Hannah’s site it was growing in 20 cm of water!
Finally, an aquatic botanical challenge for the remaining season, and next year: please look out for possible occurrences of Baldellia repens (Creeping Water plantain). It’s now known that this does occur in Ireland and that some plants previously recorded as Baldellia ranunculoides (Lesser Water-plantain), may in fact be B. repens, as Paul Green found at Lough Leane, Co. Kerry earlier this summer. The differences are subtle, but B. repens seems to favour more base poor habitats, and is more prostrate, with creeping stolons, larger, sparser flowers and a longer flowering period. But a word of caution: both species are quite variable, and may also hybridise!
Bridget Keehan Ireland Officer
who joined BSBI in 1972 and remained a member for some 40 years. Dr J. Timson of Manchester, a member for 66 years, and referee for Polygonum 1973–76. Mr B.A. ‘Jesse’ Tregale of Bradford, a member for 36 years. Prof. B.A. Whitton of Durham, a member for 73 years. Mrs A. Young of North Shields, Tyne and Wear, a member for 27 years.
Chris D. Preston, Obituaries Editor cdpr@ceh.ac.uk assisted by the Membership Secretary, Gwynn Ellis Date of compilation 26 August 2025.
Solution to Botanical Crossword No. 35
ACROSS: 1. GINKGOALES 8. PETIOLE 9. IMAGE 10. SORI 11. SEBESTEN 13. PILAU 14. OLLAS 16. PERICARP 17. IRIS 19. WHEEL 20. OFF SITE 22. GOOSEGRASS DOWN: 1. GAPES 2. NATURAL ORDER 3. GEO 4. ABELES 5. ERIGERON 6. DACTYLORHIZA 7. BEANOS 12. CURCULIO 13. PAPAWS 15. DROOPS 18. STEMS 21. FIG Crib ACROSS: 1. GINK/GO/ALES 8. PET/I/OLE 9. I<MAG>E 10. sorry 11. SE<BEST>EN 13. anag PAIL + U 14. reverse SALLO(W) 16. charade 17. rev SIRI 19. WHEE/L 20. anag OE STIFF 22. charade DOWN: 1. G(R)APES 2. 1 across is an example of an order in Nature 3. larGE Organic 4. anag (U)ESABLE 5. anag RIO GREEN 6. anag I HAD CRAZY LOT 7. BEAN<O>S 12. CUR<CUL(L)>IO 13. PA/pause 15. droops 18. double definition 21. rev GIF(T)
REVIEWS
Compiled by Clive Stace, Book Reviews Editor Appletree House, Larters Lane, Middlewood Green, Stowmarket, IP14 5HB cstace@btinternet.com

Trees of Britain and Ireland
Jon Stokes Princeton University Press, Oxford, 2025. Pp. 360, with numerous colour photos, drawings and maps; pbk £20. ISBN 9780691224169
When is a tree not a tree?
That’s the question I asked myself after reading Trees of Britain and Ireland by Jon Stokes, part of the WILDGuides series. On first impressions, this is a colourful, informative, pocketsized book with numerous photos of leaves, twigs, buds, fruits and flowers along with visual keys and notes on identification. There are interesting though succinct sections on native species, tree biology, woodland management techniques, tree habitats, history of trees in the landscape and importance of ancient trees, all within a mere fifty pages. The remainder of the book, totalling 360 pages including the index, is mostly devoted to the graphical identification of the species covered: 113 native, 190 common non-native species and subspecies in total.
The top edge of each species account is colour-coded according to whether native, archaeophyte, neophyte or hybrid. Species accounts are also furnished with distribution maps based on the BSBI’s Plant Atlas 2020. But, surprisingly, many of the taxa covered are not really trees but, in answer to my rhetorical question, shrubs. There is of course no strict botanical definition of a tree, though there is a consensus. Whereas here, somewhat vaguely, a tree is defined simply as ‘anything that has a self-supporting perennial woody stem.’
Many of the species included are at least debatably trees; others clearly not. For example, Dwarf Willow (Salix herbacea), considered in the introduction to be a good example of a ‘very small tree’ is later classed as a ‘low, prostrate, many branched shrub.’ Numerous other shrubs are similarly included although, for example, roses (Rosa) and brambles (Rubus) are omitted because they are shrubs! This makes the species selected seem somewhat arbitrary and including so many shrubs leaves less space for, let’s say, conventional trees.
But what of the book as a whole? The main emphasis is upon identification using annotated photographic rather than textual botanical keys, technical language being kept to a minimum. Native and naturalised species are given full descriptions with detailed photographs of key features, whereas most non-native species
are covered only briefly. The taxonomy is mostly current with only a few obvious exceptions. For example, Nootka Cypress is now Cupressus nootkatensis, not Xanthocyparis nootkatensis Native whitebeams (Sorbus aria agg ) are covered admirably, as are other complex groups such as elms (Ulmus). But the same cannot be said for non native whitebeams or rowans which are poorly covered given how frequently they occur. In fairness, the introduction to the identification section does state that many planted trees will not be in the book. But whether a tree is ‘naturally occurring’ (presumably meaning self seeded or naturalised) is not always obvious in the field where long established specimens in, say, former plantations or neglected gardens, are concerned. I am mildly disappointed at how few hawthorns other than the native Crataegus monogyna and C. laevigata are listed. Oaks (Quercus) are reasonably well covered, as are willows (Salix) despite the bizarre inclusion of dwarf shrub species. Pines (Pinus) are also well covered, spruces (Picea) and firs (Abies) not so. This variability in coverage is common throughout the book, which I suspect may cause some confusion.
Overall, this is an informative, visually attractive, portable book that is marred by species coverage (or a misleading title?). For that reason, I couldn’t recommend it to the serious field botanist. But for the tree enthusiast who does not want to
delve into a Flora or is intimidated by dichotomous keys and botanical terminology, or those simply interested in trees and want to learn more, it is a pleasant and informative alternative to the large catalogue of tree books currently available.
Cameron S. Crook

Restoring the Wild
Donald MacIntyre Crowood Press, Marlborough, Wilts, 2024. Pp. 272, with numerous colour photos; hbk £24. ISBN 9780719844386
Anybody who has had even the most superficial involvement with the recreation or enhancement of speciesrich grassland in the UK will be familiar with the work of Donald MacIntyre, either directly or through his seed company Emorsgate. Without him our ability to recreate convincing semi natural habitats, especially grasslands, would be far poorer. Grasslands are the principal focus of the book and, although other habitats including woodland are briefly touched on, this is primarily a guide to grassland restoration.
Restoring the Wild is a distillation of fifty years of experience as a pioneer of
habitat creation and restoration. It is however much more than just a technical manual. Written in an engaging and personal style it is also an excellent read that would be very accessible to non specialists with little botanical or land-management expertise. An outstanding feature of the book is the liberal use of photographs, most of which were taken by the author.
The book starts with a review of the principles of habitat creation, leading on to a necessarily brief account of grassland ecology and legislation and the pressures, agricultural and otherwise, that face the UK’s grasslands. This is permeated throughout with a deep concern and understanding of the ethics and desirability of restoring semi natural habitats to full ecological functionality. Appropriate seed sourcing is a priority. The potential for habitat recreation is considered within the contemporary agricultural context, but also includes the role of local authorities in improving urban and suburban ecology and the opportunities that we all have in our gardens. He is happy to be critical of the widespread use of alien species in urban landscaping, a practice that is frequently counter-productive and sometimes dangerous.
The real meat of this book is, however, in the detail of technique and practice. This is considered from scratch, from preliminary vegetation survey and soil analysis to how these affect the choice of methods for restoration, and indeed whether restoration is even necessary. There is a review of the techniques available to the land manager for preparation, collection and introduction of plant material and ongoing management, with examples drawn from the author’s extensive
experience. More than a third of the volume consists of 221 detailed species accounts, giving information on identification, seed collection and cultivation. This section will be of immense value to the practitioner.
There are of course some criticisms of a book that ranges so widely. The book is derived very much from one person’s experience with a bias towards the conditions of his home locality. There could for example have been more discussion of the use of green hay as a means of introducing species, and how different techniques might be more suitable for different geographical conditions. Upland grasslands for instance are dealt with very briefly. Yellow rattle use is considered uncritically, without discussion of other methods for reducing the growth of more competitive species and ways of reducing soil nutrient (in particular phosphate) levels. The species section includes a couple of species such as Adonis annua and Ranunculus arvensis which might raise an eyebrow, and there are some others such as Geranium sylvaticum which perhaps should have been included. There are a few typographical errors in plant naming, but these do not detract from overall readability. These are all small quibbles when compared to the overall quality and utility of this book. If there are points of disagreement then these should act as a stimulus to more research and sharing of experience. We have here the results of fifty years of groundwork of the first order, and it should be on the bookshelf or in the tractor cab of anybody with any interest in the management or creation of grassland.
Phil Wilson

Harrap’s
Wild Flowers. Second Edition
Simon Harrap
Bloomsbury Wildlife, London, 2025. Pp. 496, with numerous colour photos; pbk £20. ISBN 9781399418904
My dream is to have an identification guide light enough to carry and which accurately and efficiently enables naming of every plant species that I may encounter. Harrap’s Wild Flowers aims to offer such a guide to our wild flowers, described on the back cover as ‘great for beginners … essential reference for experts’, though ferns, grasses, rushes, sedges and most aquatic plants are omitted, critical groups are treated only briefly and relatively few aliens are included. The two main features of this guide differentiating it from such as Rose’s Wild Flower Key or Streeter’s Collins Wild Flower Guide are the absence of a key and the use of photographs instead of drawings. ‘How to use this book’ is kept to two pages, followed by a short glossary and explanation of the layout of the 1,225 species’ accounts.
The book’s cover, with a gorgeous photo of Grass-ofParnassus, encourages the user to dive in. The reader is advised that identifying flowers is not
easy and a lot of flicking between and dipping in may be required. To reduce this, Harrap suggests beginners start by getting to know plant families. However, users are not told how to find key features of different families and unfortunately one of the suggested ‘major families’ is ‘mint’, which of course belongs to the dead-nettle family. I fear such limited guidance may cause frustration.
Instead of a key, there are photos of groups of species on the inside covers. These are helpful for groups with distinctive flowers. However, at 2.2 cm high these ‘starter’ photos are small, often do not show leaves and give no idea of scale. Inevitably, some of the photos show only a small part of a plant: the ‘willowherbs’ photo is of an individual flower, not the inflorescence; the whorled leaves of ‘bedstraws’ are hardly visible. Even once you work out that a plant is in the ‘cabbage and cresses’ group, there are still 27 pages to work through.
The species’ accounts themselves are three to a page; each includes one to three photos, a description of features, with key features in italics, and a distribution map. I am in awe of the work to get good photos of so many species, but the main photo is able to show only one plant, in which some aspects of the plant may be in shade or not shown. To get an idea of scale, the user must read the small print. In contrast, drawings of plants can show the key features that make a plant a particular species. I love the inclusion of distribution maps from the Plant Atlas 2020 project: they both give an idea of whether one’s identification is likely and enable a snapshot appreciation of the species’ abundance and ecology.
Clearly there is a demand for the book as this is a second edition. However, I feel this guide leaves beginners a lot of work to do. For the more expert, the omission of families and some species limits its value. I think drawings work much better than photos to illustrate features, especially when space is limited, and I prefer a key that, once grasped how to use, provides a methodical process to reach a conclusion. I might use this book to look up a photo of a species, but these days I would probably get a better selection on my phone, which is also much lighter to carry. I hope, though, that the use of distribution maps becomes the norm.
Jo Jones

Europe’s Alpine Flowers
Bob Gibbons
Princeton University Press, Princeton & Oxford, 2025. Pp. 496, with over 1800 colour photos, pbk £25. ISBN 9780691230788
The appearance of a new book on European alpine flowers is a notable event. Although the geographical scope of the coverage can easily be defined on a map (in this case from the Pyrenees
and Carpathians northwards to Britain and northern Scandinavia), what constitutes ‘alpine’ cannot. Certainly altitude is of little help, because this varies immensely with latitude and even on opposite sides of one mountain. The author makes his own judgement, taking as a ‘starting point … plants that regularly occur above 1,000 m in the Alps’. Despite the fact that 1,000 m is well below the lower limit of what our Continental colleagues would consider as ‘alpine’, it would be pointless and churlish to question the author’s wisdom, but better to accept his decision and be grateful for the fact that he has provided an idea of his thinking. One, I believe unique, feature of Gibbons’ book is that it attempts to cover all species in this alpine zone. So the familiar alpine species of gentians, primulas, orchids, etc. are joined by common lowland plants, often weeds, that climb above 1,000 m, e.g. Stellaria media, Lamium purpureum and Bellis perennis, but not Senecio vulgaris (although this reaches 2,200 m in France). Moreover, the lower limits of altitude are often breached, e.g. by Primula scotica, which probably does not attain 100 m. As implied above, this is not intended as a criticism, but is something for the user to bear in mind. A different author would have both omitted and added to the species covered in this book, but would have been open to the same objections.
In all, just under 1,800 species are covered. To put this into context, it has been estimated that there are about 4,500 species in the Alpine Chain alone, i.e. excluding the Pyrenees and Scandinavia. However, a good proportion of the species not covered either belong to critical groups which are not reliably
distinguished with the aid of a picture book or are extremely local species (or both). Moreover, ferns, grasses, sedges and rushes are completely excluded. The species entries cover 438 pages, mostly 4–5 to a page, each one with a representative photo and text with a brief description and a summary of habitat and distribution, etc. Before these accounts there are introductory chapters on ‘how to use this book’, notable alpine habitats and localities, very helpful tips on identification, the names of plants and a glossary. The author is careful in pointing out when a group is taxonomically difficult, where more specialist literature might be needed, and in emphasizing the distinction between very similar species.
To most British botanists Bob Gibbons is best known as a superb plant photographer, and his recent death aged 74 is much lamented. At the start of the book there is a two page tribute to Bob by Peter Marren and Richard Mabey. Bob’s photos are a familiar sight in many BSBI publications as well as in his own and others’ books. According to the tribute Bob died ‘just as this book was reaching the editing stage’, and unfortunately the book shows signs of the absence of his oversight at the end. Most disappointingly, the reproduction of many of the photos does not do justice to the presumed excellence of the originals. To my mind, many of them are too dark, sometimes making the details difficult to make out. This applies particularly to blue flowered species, e.g. Veronica spp., Aegonychon and Lavandula, and those where the plant merges into the background, e.g. Spergularia rubra, Neottia cordata and Bupleurum spp. Such photos do not help in
identification and I feel sure that the author would have upgraded them had he seen the proofs. There are other imperfections that could have been corrected. Several of the captions have been applied to the wrong photo: without any systematic searching I noted captions swapped between two species on pages 254, 261, 448, 456 and 462. Confusingly for British botanists the English names used for some plants are not those commonly used in our literature, e.g. Melampyrum sylvaticum, Cardamine bulbifera and Hornungia petraea. The nomenclature is said to follow the World Flora Online, but there are departures, e.g. the generic boundaries of Saxifraga and Sedum. More important than the above, however, is the fact that I detected very few factual errors, a tribute to the author’s knowledge over such a wide range of plants. I will point out only one. The diagnostic difference between the two Laburnum species lies in the morphology of the fruits; this is not mentioned, and the differences cited (mainly pubescence) are not reliable.
Despite its minor mistakes, which are inevitable in a work of this scope, Europe’s Alpine Flowers is the best available on the subject and will become an essential companion in the field. Viewed as a whole, the deficiencies are negligible compared to the treasure of information provided. The ability to scan through a long series of photos of alpine genera, very attractive but often very difficult to identify (e.g. Gentiana, Primula, Dianthus, Pedicularis, Phyteuma and Campanula) is worth the purchase price alone.
Clive Stace

Brambles of Scotland
Angus Hannah
Botanical Society of Britain and Ireland, BSBI Handbook No. 25, Durham, 2025. Pp. 220, with numerous colour photos and maps; pbk £20. ISBN 9780901158628
With 350 or so apomictic Rubus species in the British Isles I was anticipating another weighty tome on my doorstep, but as Brambles of Scotland focuses on the 56 ‘resident’ Scottish species it comfortably sits beside most of the other BSBI handbooks, and is lightweight enough to be carried into the field – a benefit as brambles rarely make the most cooperative specimens and it helps to be able to take the book to the bush rather than vice-versa. It was also pleasing to see a return to the colourful monochrome lino-cut style covers of the earlier BSBI handbooks.
The introductory pages provide general information on Rubus, including ecology, recording and the history of batology; there is novel discussion on dispersal and distribution, and clarification of some of the quirks of the genus, such as why new species must have a large enough range before they should be named. A ‘gallery of characters used in identification’ provides discussion
of the taxonomically important parts of brambles, and for the first time couples this with detailed photographs of each. This alone is a very useful resource for a budding batologist and makes the book relevant well beyond Scotland.
Two keys are provided; a dichotomous key to 48 species and a multi-access key to 51 species. It is a little odd that some resident Scottish taxa are not treated in the keys. Given the reduced number of species in Scotland the traditional use of artificial series is more or less dispensed with, allowing a refreshing alternative to the well worked standard keys. I tested both keys on a pile of Scottish herbarium specimens and arrived at the correct names more often than not. However, I found the dichotomous key more successful, quicker and more pleasing to work through. Most specimens keyed well but others needed to be coaxed through the key knowing the answer, and I was unable to get a few to a satisfactory conclusion. The presence of anther hairs is used early in the keys; while this is a good discriminative character, they are notoriously inconspicuous and also deciduous, so perhaps are better used at the ends of keys. But, generally, the couplet choices are straightforward.
Just over half of the book is dedicated to the species accounts. Each species is given a double page spread including a full page of five to eight photographs, and a distribution map embedded in the text. Many of the species illustrated are widespread British brambles and I found myself flicking through the book as a quick reference for those species on my patch (eastern England) rather than digging
out photos or descriptions. The lavish use of colour photographs is unprecedented in a book about brambles and is to be much commended. It allows names arrived at in the key to be confirmed in the field, which to date has not readily been the case (unless with good smartphone reception and one of the bramble websites), and as apomicts differ little across their range it opens the possibility of visually matching a bush and pictures in the first instance (and then checking key features). Unfortunately some photographs have lost quite a bit of definition, particularly on the macro shots. More significantly Rubus subinermoides is illustrated with images of a very different taxon; the discrepancy is discussed in the text, but the images are certainly not of Rubus subinermoides as it is known in its south-east England core area, differing in several diagnostic characters. This bramble would have been better dealt with under a working name. Sometimes the illustrations would benefit from the addition of photos of key characters (e.g. the pink anther sutures of Rubus amplificatus). The text for each species entry discusses the diagnostic features in an accessible way, while touching on distribution, ecology and similar species. Further rare and adventive species are illustrated using herbarium specimens in an appendix.
In summary, this is a brilliant and beautiful book which will be a useful reference especially in Scotland but also beyond its geographic scope. A photographic guide to brambles has been much anticipated and I am sure it will greatly support the increasing interest in this prickly genus.
Alex Prendergast

Identification Guide to the Non-native Invasive Plants of Britain and Ireland
Fran Giaquinto & Phoebe O’Brien Pelagic Publishing, London, 2025. Pp. xiv + 154, with numerous colour photos and maps; pbk £29.99.
ISBN 9781784275648
There is a need to raise awareness about the impact of invasive non-native plants (INNs) on biodiversity. Legislation has done little to control spread of these species despite millions spent on efforts to eradicate them. ‘Know thine enemy’ said Sun Tzu.
This illustrated guide provides detailed descriptions of some of the worst offenders, with distribution maps, relevant legislation and how to distinguish them from similar species. The quality of the photos is generally good, and it is nice to see illustrations of littleknown taxa like Luma apiculata, Juncus planifolius and Rubus armeniacus. The inclusion of half a dozen seaweeds is an important reminder that marine habitats are equally threatened by invasive taxa. I liked the description of Japanese Knotweed as ‘vigorous, especially when disturbed’ and was intrigued to learn that the scent of Skunk cabbage flowers is reminiscent of cannabis.
To make the book more accessible to non experts, the accounts are arranged by size and growth form. If you find an invasive tree, but have no idea what it is, you can flip through the section on trees to get close to an identification (if you are lucky enough to have one of the five trees included). Oddly, Rhododendron and Buddleja are classed as trees, not shrubs.
The cover text claims it is a ‘practical tool … for everyone interested in the protection and sustainable management of our countryside’, yet it offers no solutions for the control of these plants and does not list resources or publications that might help. All it suggests is that we don’t plant them in the first place (too late now the horse has bolted!) and report them to British and Irish databases.
The definitions of native and invasive depend on many factors: there are many plants that are only native to some parts of Britain and Ireland e.g. Clematis vitalba native to Britain but not Ireland (it is a pity the map for this species does not show status, although maps showing this are available on the BSBI’s Plant Atlas 2020). Some species are only invasive where climatic conditions are right, e.g. Sarracenia and Gunnera that are only problematic in wetter parts of Britain and Ireland. The ‘lookalikes’ often include other invasive species, and in some cases the featured species is less frequent than the ‘invader’, e.g. Smyrnium perfoliatum over S. olusatrum. For butterburs, Petasites japonicus is given pride of place whereas P. pyrenaicus is the only truly invasive species. It is odd that Arundo donax, Persicaria perfoliata and Cortaderia jubata all get double page spreads despite not being established in Britain or Ireland. There are some obvious omissions,
e.g. Acer pseudoplatanus, Quercus cerris, Q. ilex, Populus alba, Ludwigia spp., Lemna minuta, Solidago canadensis, Pentaglottis sempervirens, Euphorbia amygdaloides subsp. robbiae, Erythranthe spp and Symphyotrichum spp.
There are some signs that the book was put together quickly, or not thoroughly proofread: a repeated sentence in the description of Giant Hogweed; a table of dispersal methods that includes a dozen native ‘lookalike’ taxa; and the ‘at a glance guide to flower colour and leaf shape’, which takes up five pages at the beginning of the book and is a waste of space with tiny leaf images at various scales. In the glossary, the definitions for ‘peduncle’ and ‘palmate’ are quite wrong. Buddleja does not selflayer or root from fallen branches, but its spread by wind blown seed is highly successful, although most of the sites it colonises are urban.
Despite legislative attempts to pin down lists of INNs, the criteria are not static. Opinions on nativeness can change, as shown by the recent demolition of the ‘Lusitanian flora’ in Ireland by Sheehy Skeffington et al. (e.g. British & Irish Botany 5: 221–251, 2023). The invasiveness of species can also change with climate warming or as new dispersal vectors enable them to reach different habitats. There is very little in this book about horizon scanning for the next generation of invaders waiting at the garden gate or across the Channel.
The book does a good job of describing an eclectic mix of invasive species, but is a bit thin on detail about their invasive potential and says nothing about how we might tackle them where they occur.
Martin Sanford

A Field Guide to Urban Plants
Alexandra-Maria Klein & Julia Krohmer
Pelagic Publishing, London, 2025. Pp. 144, with numerous colour photos; pbk £17.99.
ISBN 9781784274740
Despite recent and rapidly increasing interest, urban botany remains an overlooked area of scientific study and exploration by the natural history community. Non-academic publications examining the intrinsic value and interest of the botanical denizens of our streets, railways and canals are almost unheard of, which is unfortunate considering the desperate need to connect people to nature and the increasingly urbanised nature of our lives. Klein and Krohmer have attempted to address this void by producing an accessible work that aims to evoke interest in urban plant life through a series of short biographies detailing basic identification tips alongside snippets of information about the plants that are aimed at triggering the interest and imagination of someone taking their first steps into natural history. The biographies are supported by photography and artwork to enable the reader to visualise the
ideas being discussed as well as to identify the plants in question.
The opening brief chapters of this work outline why the authors feel there is a need for ‘building awareness’ about the lives of urban plants. Not surprisingly, the concept of ‘plant blindness’ (the inability, or unwillingness, of society to see plants as an intrinsic part our world and wellbeing) is a central element of the authors’ rationale for the book. They also draw out the special character of urban ecosystems as well as the personal, emotional benefits of gazing at plants. Helpfully and generously, throughout the book the authors direct the novice to organisations such as the BSBI.
The main body of the work is arranged around the biographies for the 108 species that the authors have selected. Sensibly, they are diligent about informing the reader that this work is not comprehensive and that urban botanising is full of surprises. The plants are arranged artificially, principally by colour but with further sections relating to ‘Woody plants’, ‘Grasses’, ‘Ferns’ and ‘Mosses and Liverworts’. Each species account is headed with the plant family and scientific and standard English names for the plant; alongside these are jaunty bylines that aim to draw out an aspect of interest. These bylines have the tone of a hipster coffee shop and sometimes expose the, at times, not entirely smooth translation from one language to another; a choice example of this is on page 25 where Thyme-leaved Sandwort (Arenaria serpyllifolia) is described as ‘scruffy asterisms’ – an idiom that has not quite made it unscathed into the anglophone realm (the book was originally published in German in 2023 as Die Wächst in Deiner Stadt). The
main body of the text details the aforementioned snippets, many of which focus on the ethnobotanical properties of the plant. Finally, each entry is footnoted with information detailing the plant’s morphological characteristics and flowering times. Alongside the text, each plant is illustrated with original artwork and photography. Most of these images are of reasonable quality and demonstrate, via sketch-note style typography, beginner friendly identification features.
Accepting that this work is directed at the beginner, I have very few significant quibbles, but it is obligatory to note these down. I suspect there are one or two misidentifications in the images; notably the photograph of Viper’s bugloss (Echium vulgare; p. 107) appears to depict Purple Viper’s bugloss (E. plantagineum) or a similar taxon. Importantly, it would have been advisable to have co opted the expertise of a British or Irish botanist to evaluate the text in relation to our floras; a notable error arising from a lack of detailed familiarity with our urban flora is the inclusion of Sand Spurrey (Spergularia media) (p. 88) as a common urban plant. In this country, certainly in southeast England at least, this plant is generally uncommon and not associated with urban habitats.
This book is an ideal gift for the beginner botanist; while it has some faults, they are faroutweighed by the accessible and engaging nature of the work.
Mark A. Spencer

Urban Plants
Trevor Dines
Bloomsbury Publishing, London, British Wildlife Collection 15, 2025. Pp. 384, with numerous colour photos; hbk £40. ISBN 9781399407496
Arguably we are currently in a golden age of wildlife publishing. Despite the electronic pocket encyclopaedia that most of us now carry there is still a market for an entertainingly written, well illustrated book that wears its erudition lightly. The rejuvenated Collins New Naturalists have been meeting this demand for over twenty years and the success of the Bloomsbury British Wildlife series over the last decade has shown that there is still a wider demand for such publications. The latest edition to this latter series is Urban Plants by Trevor Dines. Most humans now live in urban environments. Society is beginning to recognise the value of plants in our everyday lives both from a physical and a mental perspective (though we botanists have known it all along), hence understanding our urban flora is important not just for the plants but for ourselves too. The general reader will look hard for anything on the subject. Gilbert’s
Ecology of Urban Habitats (1989) and Darlington’s Ecology of Walls (1981) are long out of print. David Goode’s more recent New Naturalist Nature in Towns and Cities (2014), while excellent, has a distinctly animal bias to this critic’s eyes. Hence this is a timely book.
The challenge for an author choosing to write about an urban flora which is a complex mix of both native and non native species is to develop a framework that prevents it becoming a simple list of facts. Dines does this with a very clear structure. Part One is the broad historical perspective; where has our urban flora come from? Part Two explains the ecology of the urban environment and a more detailed consideration of the various origins of the urban flora. Part Three is a walk around five key habitats (pavements, walls, derelict spaces and waste ground, urban grasslands and street trees). The final part covers the future of urban plants.
The ecology section is the scientific underpinning of the book. Urban plants have a stressed existence through heat, drought, pollution and a variety of soils. These influencing factors are each covered in turn with enough depth to explain them fully but without compromising the science. Deftly chosen examples illustrate the points. All readers will surely have their own ‘Oh, I didn’t know that’ moment. (Two of mine are that Middlesbrough city still retains some ancient grassland and that children once made pea shooters from Giant Hogweed!)
Each of the ‘walk’ chapters has a clear structure too: a definition, a revisit of the relevant ecology, though always with something new rather than a reiteration of the earlier section, followed by detail of the plant communities.
While a wall might be easy to define, some of the other habitats are more difficult. The author doesn’t shy away from these challenges. I particularly liked the coining of a new term for waste ground and derelict land as ‘urban fallow’. Hopefully this two word encapsulation of such a valuable habitat will become a commonplace term. The description of the successional stages on this urban fallow; pioneer Oxford Ragwort followed by Rosebay Willowherb Tall herb stage, leading to the Coarse Grassland stage culminating in the Buddleja Scrub woodland stage is novel, though familiar to those of us who botanise on such sites, and deserves wider research.
I think there are two criteria to measure the success of such a publication: the book itself, and its wider impact. It is certainly entertainingly written with a strong narrative that combines scientific context, original observations and the writer’s own knowledge and experience. The text is supported by excellent illustrations and Carry Akroyd does her usual marvellous job of the cover artwork. The book is recommended without reservation.
The wider impact is impossible to judge at this stage. Nevertheless I predict the book will generate great enthusiasm for its subject. I couldn’t help but view the flora on my local estate in a new light and I anticipate an increase in recording of the urban flora. Hopefully an additional legacy will be the recognition of the value of such sites from both a human and plant perspective.
Paul Ashton







ALL our profits are donated to nature conservation
natural history holidays
small friendly groups
relaxed pace
expert leaders
2026 destinations include:
Crete: spring flowers
Estonia: Baltic spring wildlife
Cyprus: orchids & ancient sites
Morocco: coast and mountains
Lesvos: spring flowers & easy birding
France by train: Dordogne, Vercors, Cevennes
Chile: coast & Atacama, with Sarah Lambert









