




By Lauren JohnSon
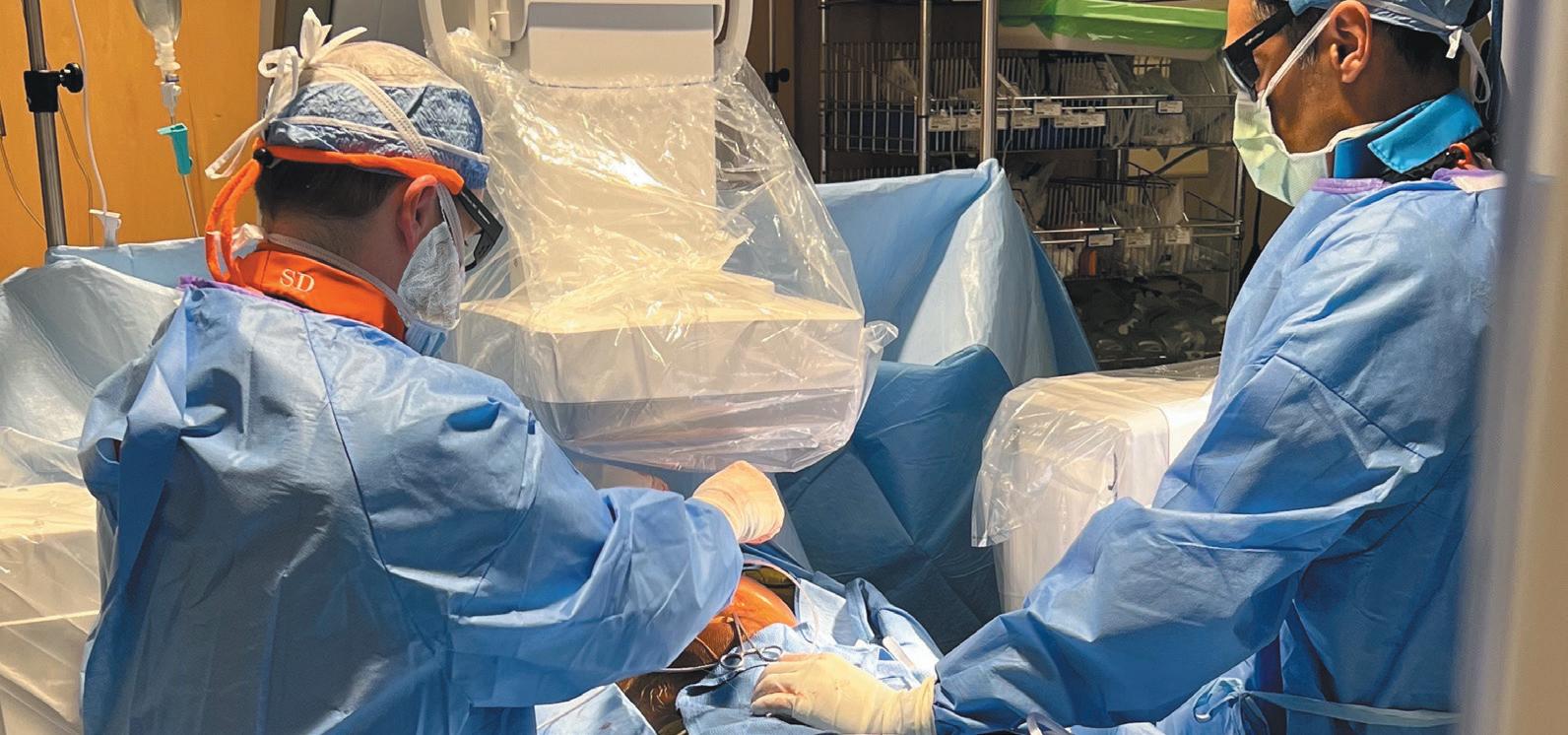
In May, Sean Dunn, MD, a cardiac electrophysiologist at UAB, became the first physician in the state of Alabama to successfully implant the newest extravascular defibrillator technology, which will be used to treat cardiac patients. This device provides another option for patients at risk of heart failure, arrhythmia and sudden cardiac arrest.
The Aurora Extravascular Implantable Cardioverter Defibrillator (EV ICD), developed by Medtronic, is the first of its kind because the lead is placed under the breastbone instead of through veins and into the heart.
“Traditional defibrillator systems have leads implanted through the blood vessels,
(CONTINUED ON PAGE 4)

By Laura Freeman
In exploring strategies to help new ideas move from concept to marketplace, you can learn a lot about the nature of creativity by considering how astrobiologists search for life in the universe. It all comes down to the right environment.
Billions of stars in our home galaxy multiplied by the vast numbers of new galaxies now visible through the Webb telescope might make the choice of where to start seem overwhelming. But scientists improve their odds for success by looking for places that combine the factors pres-
ent where life has already managed to arise.
Just as stable stars with moderate size planets in the goldilocks zone are more likely to have temperatures for liquid water, light for photosynthesis, and enough gravity to hold an atmosphere without crushing small molecules, you can take a similar approach to building an environment to nurture innovation. With that in mind, recent reports from the Brookings Institution, Hoover Institution, and Deloitte conclude that the strength of Birmingham’s medical
(CONTINUED ON PAGE 8)






At the heart of one of the most highly regulated industries in the nation, health care facilities and providers face challenges that draw attention and resources away from their mission to provide quality patient care.
Burr & Forman’s health care team works alongside you to address and anticipate your needs by providing preventative guidance and pragmatic solutions, enabling you to focus on providing care and achieving healthy outcomes.
Alabama Health Care Team
Birmingham | Mobile | Montgomery
Jessie Bekker
Howard Bogard
Richard Brockman
Kelli Carpenter Fleming
Jim Hoover
Catherine “Cat” Kirkland
Garrett Lucey
Angie Cameron Smith

By Laura Freeman
“It isn’t a federal regulation, but if you want to be able to accept credit card payments without fines or undue legal and financial risks, it’s important to be diligent in making sure your office is in compliance with current payment card industry data security standards,” Janet Day, senior healthcare advisor for Kassouf & Company, said.
“DSS 4.0 has been implemented over the past two years and became fully effective a couple of months ago. A few tweaks are now being added under 4.1. Complying with these security measures are part of the contract you signed when you were approved to accept card payments. Failing to meet these standards, even if you simply misunderstood or relied on incorrect advice from someone, could cost you thousands of dollars and possibly affect your ability to accept cards for future payments.”
The first set of standards was established in 2004 when American Express, Discover Card, Mastercard and Visa
came together to create a security plan to counter the efforts of increasingly ingenious thieves who are continually coming up with new ways to steal information remotely through data systems. The security standards have continued to evolve with fresh counter measures to block whatever new routes cyber criminals try to use.
These security requirements apply to all organizations that accept card payments using a swipe or similar technology, take card payments over the phone, keep card numbers on file or transmit them. In addition to being compliant, third parties that your digital and communications systems interact with should also be compliant. That includes practice management software, accounting programs like Quickbooks, online banking services, collections services and other card services and third party reimbursement systems.
“The security requirements focus on preventing breaches, fraud, and keeping private data safe,” Day said. “In addition to maintaining and regularly updating firewalls and protecting against malware, you need multifactor authentication. You should also have a policy that keeps passwords strong and regularly updated.”
Encryption of sensitive data, and disposal when it’s no longer needed are also important. You will also want to

Alabama
Alabama
Alabama Pain Physicians is celebrating our 16th anniversary of providing the best comprehensive pain management care to the greater Birmingham area. We’ve worked hard to become the practice we are today. Thank you to our staff, patients, and referring physicians for your support over the past 16 years!

monitor for suspicious activity and set up and document a vulnerability assessment program.
“You’ll need to find a service that can perform an annual vulnerability scan,” Day said. “An annual risk assessment is also essential, and this is where many organizations make a misstep that can lead to problems later. Large companies like big box retailers that accept an enormous number of card payments can employ certified experts who can examine every aspect of their card payment system, and verify that it is in compliance with all requirements. However, becoming certified is a long and arduous process, and someone with that expertise can be very expensive. For a practice that accepts a relatively small volume of cards, the costs may not fit into the budget. However, it
is possible to find people who are highly trained in this area who may not yet be certified, but can offer good advice.
“Where organizations with a smaller digital footprint tend to get into trouble is that they may hire freelance IT people to set up their systems, which allows them to save money. When the form for the annual assessment comes in, the person responsible for compliance may have other responsibilities to manage and may not be that knowledgeable about the more in-depth issues. So they send the form to their IT guy, who is an expert in hardware but may not know that much about the specifics of how their client is managing compliance. They may assume their client knows whether they are in compliance. They just sign the form and send it back. Whoever is responsible for returning the form sees the IT signature and thinks they must know if they are in compliance. They return the form unaware that they could be vulnerable to hacks, fines, and legal costs from patients whose card information is compromised.”
Regular training for every member of staff who has access to card information, and staying current on training new hires, are a good first step in preventing problems. Also, limit access to card numbers to those who have a need to know. Think about who can see card



Security, continued from page 3
numbers. Is this someone you would trust with your wallet?
“We don’t want to stress anyone, but we do want you to be sure you aren’t left vulnerable by misconceptions and myths. You can go online and find spreadsheets from the card companies you do business with that list the most common questions, answers and data points you are likely to need to understand. It’s called the prioritized approach tool for PCI DSS v4.0,” Day said.
Reviewing spreadsheets may not be the most exciting way to use your time, but when you learn what you need to know, you can relax, and get back to the work you enjoy.

which can have lots of issues as patients age. Leads implanted through blood vessels are at risk for fracturing or even becoming dislodged so we’ve tried to develop systems that don’t have these limitations,” Dunn said. “The first device that came out was called a subcutaneous ICD, which was a lead implanted under the skin on top of a breastplate, but that also had limitations. Whenever you’re outside the chest, it requires a lot more energy, so it’s a bigger device.”
Because the EV ICD is implanted under the breastbone and on top of the heart, it’s capable of sending energy to the heart in a smaller, compact form. This new technology can also pace the heart, which can potentially stop an arrhythmia and correct a dangerously abnormal heart rhythm.
“The typical treatment is to shock the heart back into rhythm, which can be uncomfortable, but if we’re able to pace the heart, we can pace the heart faster than the arrhythmia and stop it in a painless fashion. This is one of the nice parts about this device,” Dunn said.
If pacing the fast heartbeat is unsuccessful, the device would then deliver a shock to reset the heart’s rhythm. For a slow heartbeat, the device can stimulate the heart through pacing to get it beating again.
In the EV ICD Pivotal Study, con-


ducted by Medtronic, researchers recorded that the Aurora Ev ICD had a success rate of 98.7 percent. A total of 299 patients received an implanted system, and according to the study, all discrete spontaneous arrhythmias were successfully treated.
In addition to a high success rate, this device is also expected to have a longer battery life than other ICD systems. The subcutaneous ICD battery life typically lasts about seven years while the Aurora EV ICD battery is expected to last 11 years.
“I think this would be a great option for people who are at risk for an infection, and we want to try to avoid being in the bloodstream, or perhaps for those patients who don’t have blood vessel access to the heart. It’s also a great option for younger, more active patients who don’t
want to have the long-term kind of risks related to having things inside the blood vessel,” Dunn said.
Implanting the device involves a minimally invasive procedure where physicians insert it below the left armpit under the breastbone, which has shown to have good cosmetic results, according to Dunn.
“There’s a growing population of people with cardiovascular disease, especially in the South, so the general trend is that more patients are requiring therapies like these,” Dunn said. “Arrhythmias are being detected more often now as we continue to have an increase in people wearing smart devices.”
Sudden cardiac arrest (SCA), which is caused by rapid or abnormal heart rhythms, claims the lives of approximately 475,000 Americans every year. This makes SCA the third-leading cause of death in the U.S. Only one in 10 patients who experience SCA survive, according to research provided by Medtronic. The Aurora EV ICD system is the latest technology that is expected to combat these statistics and improve care for at-risk patients.
“I’m glad we’re able to offer this therapy to patients,” Dunn said. “I think it shows that UAB is really at the cutting edge and trying to offer the most advanced care to Alabamians.”
If you’re searching for new revenue sources to strengthen your practice, it’s time to partner with Seven Corners Healthcare, the largest third-party administrator of federal inmate healthcare in the country. Join our network for:



Competitive reimbursement rates
No patient copays for deductibles to pursue
Expanded revenue through a range of specialties, including orthopedics, podiatry, gastroenterology and more













By angie Cameron Smith and Sara Petermann
With changes in presidential administration often come seismic policy shifts, and policy with respect to healthcare is no exception. On May 22, the Centers for Medicare & Medicaid Services (“CMS”) announced its updated guidance policy regarding the use of the code “999999999” as a placeholder in hospital machine-readable files (“MRFs”).
According to the guidance, this update is consistent with Executive Order 14221 (“Order”), which was issued by the White House on February 25, 2025. The Order, titled “Making America Healthy Again by Empowering Patients with Clear, Accurate, and Actionable Healthcare Pricing Information,” reflects the current administration’s stance on transparency in hospitals’ pricing practices. The Order and subsequent guidance carries important implications for hospitals.
Background
Section 2718(e) of the Public Health Service Act mandates that hospitals provide to the public information on their standard pricing for items and services,

and in 2021, these pricing requirements were implemented via the Hospital Price Transparency regulations, found in 45 CFR 180. Consequently, MRFs were required for hospital charges, including innetwork provider rates, historical allowed amounts for out-of-network providers and any negotiated rates for prescription drugs. This information must be available in a consumer-friendly format on an internet website free of charge for public consumption. Previously, CMS instructed hospitals to encode “999999999” in lieu of an actual dollar amount when there was “insufficient historical claims data” to calculate a specific dollar value. However, CMS found that this practice was overused and undermined the purpose of the price transparency rules, which were

intended to increase patient awareness with respect to hospital pricing by requiring more robust disclosures. As a result, CMS updated its policy regarding the use of code “999999999” as a placeholder in the MRFs.
So, what changed?
CMS now advises hospitals to encode a specific dollar amount in the MRF for all items and services offered. CMS acknowledges, however, that there may be situations where a hospital has limited historical claims to establish the estimated allowed amount. In these instances, rather than encoding “999999999” in the MRF, hospitals must instead encode “the average dollar amount the hospital has received for an item or service, derived from the electronic remittance advice transaction data using data from items or services rendered within the 12 months prior to posting the file.”
In other words, if an item or service was used at least once within the preceding 12-month period, hospitals are expected to take the average of those prior charges and encode that value into the MRF. If an item or service was not used at all within the preceding 12-month pe-
riod, hospitals are instructed to encode a value that is their “expectation of what the charge would be for that item or service, and remark in the ‘notes’ data element that there were ‘zero instances of the items or service in the 12 months prior to posting the file.’”
The Trump administration states that increased price transparency will “support a more competitive, innovative, affordable, and higher quality healthcare system.” According to some, pricing disclosures for hospital services encourages free-market competition by allowing consumers to shop different hospitals for the best price.
Penalties for noncompliance by hospitals could range from civil penalties to public shaming, with CMS publicizing noncompliant hospitals on its website. We have seen a number of hospitals receive notification from CMS of non-compliance with the Hospital Price Transparency rules, as well as requests for Corrective Action Plans.
With respect to patients, providing complete and accurate information on hospital pricing allows for informed decisions

















Ransomware attacks are more common than ever in today's healthcare climate. Our team of healthcare information technology experts can develop a plan to keep your organization protected.
From risk assessments to our Virtual Chief Information Officer Services, we are here to thwart any cybersecurity threats that come your way.








community combined with the city’s history of manufacturing make Birmingham well-suited to become a medical device mecca.
“We have so much healthcare expertise in Alabama with lots of talented people who have great ideas. Being the best in their field may not allow these people enough time to learn about the steps needed to take their concepts into a design and prototype,” Mark Connor, Executive Director of a Hardware Park, a nonprofit 501(c)(3), said. “The legal maze of patents and regulations can be hard to navigate. If you aren’t a banker or broker, how do you know where to look for capital? It can take a lot of cash to move your prototype through testing and approval. Then, when you have a product, how do you use marketing and distribution to get it out to people?”
Business incubators bring together the elements necessary to nurture the creation and launch of new enterprises.
“Incubators in Birmingham have been successful working with pharmaceuticals and other health-related areas,” Conner said. “We saw that entrepreneurs with ideas for physical products, particularly medical devices, needed support. In addition to helping them, it gives us the opportunity to train the next generation of designers and engineers. This can also help power the expansion of manufactur

ing in our state in new sectors that offer potential for future growth.”
While other incubators may focus on workspace, Hardware Park focuses on think-space and networking. Located on Fifth Avenue North in the building that originally housed the Long-Lewis Hardware Company, Hardware Park has space where people can work on fabricating prototypes, but the campus is primarily set up for offices, hands-on education and connecting entrepreneurs with expertise.
“Our clients are able to sit with designers who can offer insights in optimiz-
and paper for better performance when they work with engineers to select materials and manufacturing strategies,” Conner said. “We put them in touch with legal teams who have been through the process, and introduce them to financial experts who can guide them through different options for raising capital and explain the advantages and alternatives. If they need marketing help, we have resources on site. We can also put them in touch with a variety of distribution channels.”
Clients already on site include OD Revive, a MedTech startup that is developing a wristband that detects opioid overdoses and calls for emergency help. Another health technology startup housed at Hardware Park, Nephsol, is working on a non-invasive, blood flow monitoring device to optimize treatment outcomes for dialysis patients. Hardware Park also has clients who are working on ideas to improve mobility devices. And Fulmer Instruments, founded by Birmingham neurosurgeon Benjamin Fulmer, MD, designed their medical device at Hardware Park.
Innovate Alabama, the statewide public-private partnership focused on entrepreneurship, designated Hardware Park as an innovate entity, and gave the organization a grant for medtech design internships for local high school and col
lege students. With this, Hardware Park officially launched the NextGen Education program, which immerses students in hands-on design, engineering, and manufacturing experiences with a strong focus on MedTech device innovation.
“Our NextGen program offers students interested in biomedical design and engineering an opportunity to work in hands-on internships. Undergraduate students from UAB, Tuskeegee and Auburn have already worked in internships using 3-D printers and other design and engineering tools,” Connor said. “Our interns learn by doing, getting experience that goes beyond a line on a resume. They get real insights in the work they are planning to spend their careers doing, and they have an opportunity to actually share their talents in areas where they can make a contribution.”
By bringing together ideas, hands on talent, resources and fostering opportunities for new jobs in the future, Hardware Park is working toward a win for Alabama. No bright idea should be left to burn out for lack of available elements that could spark it to life.























































By hoLLand & Knight’S PuBLiC PoLiCy & reguLation grouP
The Make America Healthy Again (MAHA) Commission, as established by President Donald Trump’s Executive Order 14212 in February 2025, has released its inaugural report titled “The MAHA Report: Making Our Children Healthy Again.” The Report identifies several factors it argues are driving up rates of chronic disease in the U.S. among children, including ultra-processed foods (UPFs), poor diet and lifestyle habits, environmental exposure to certain chemicals and overuse of certain prescription drugs.
Although the Report’s findings dovetail with many of the same issues U.S. Department of Health and Human Services (HHS) Secretary and MAHA Commission Chair Robert F. Kennedy Jr. has emphasized for years, the Report does not result in any immediate changes
to regulatory frameworks for the approval of food additives, prescription drugs, pesticides or other chemicals. However, the Report is likely to be used as a precursor to a comprehensive strategy addressing its findings – expected to be completed in August 2025. The EO calls for a governmental approach that deploys proactive policies as well as a realignment of food, health and scientific systems to prioritize prevention and resilience.
The Report argues that four key factors contribute to increasing rates of chronic illnesses.
• Poor Diet: The Report cites the growing prevalence of UPFs in the diets of Americans as a key driver of chronic disease rates. This includes ultra-processed grains, sugars and fats as opposed to whole foods, as
Exclusively for medical professionals, The Service has provided reliable, caring service to physicians and their patients for over 30 years.
• Change your schedule from computer, tablet or smartphone
• Operator follow-ups with patient to confirm calls are returned
• All calls recorded
• Messages delivered via pager, text, phone, fax or email
• HIPAA-compliant secure messaging
well as the inclusion of additives such as emulsifiers, binders, sweeteners, colors and preservatives. The Report discusses and criticizes the shift in dietary fats from minimally processed animal-based sources to industrial fats derived from refined seed oils such as soybean, corn, safflower, sunflower, cottonseed and canola.

• Chemical Exposure: While acknowledging the benefits of industrial chemicals in modern life, the Commission emphasizes that children are uniquely vulnerable to the health risks posed by widespread exposure to synthetic substances, particularly in early developmental stages. Childhood exposure to chemicals including heavy metals, per- and polyfluoroalkyl substances (PFAS), pesticides, microplastics, phthalates, bisphenols and other products through ingestion, inhalation and other means are also cited as a factor behind chronic disease. The Report highlights that more than 40,000 chemicals are registered for use in the U.S. – many of which are found in the blood and urine of children and pregnant women – such as pesticides, microplastics, dioxins and endocrine-disrupting compounds.
Beyond these drivers, the Report focuses on several topics, calling for several significant reforms in food systems, school nutrition programs and federal dietary guidelines. For instance, with respect to UPFs, the MAHA Commission calls for a national shift toward whole foods – which it defines as minimally processed, nutrient-rich options produced by American farmers.
• Changes in Lifestyle Habits: Lack of physical activity and increasing amounts of time spent online are also listed in the Report, specifically as a detriment to mental health, which has been seen in the form of increased stress, feelings of loneliness and anxiety among children.





• Overdiagnosis and Overmedication: The Report cites the prescription of antidepressant, antipsychotic, antibiotic and stimulant medications among children as the final, major contributing factor to rates of chronic disease. Anti-obesity, asthma drugs and vaccines included in the childhood vaccine schedule are also highlighted. The Report cites the need for additional placebobased, longitudinal and randomized clinical trials to better evaluate the impact of exposure to certain medicines on children’s health.
With respect to the growing concern over the cumulative chemical burden American children face in their daily environments, the Commission calls for a reassessment of current regulatory frameworks and scientific methodologies to better understand and mitigate the long-term effects of chemical mixtures on child health. Although some studies show associations with conditions such as obesity, early puberty, infertility and neurodevelopmental disorders, the Commission stresses that more rigorous, long-term research is needed. It also acknowledges that many of these substances are present in everyday items such as food packaging, personal care products and household dust, making exposure nearly unavoidable.


The Report concludes by outlining 10 steps the MAHA Commission will take to combat chronic disease:




1. Address “replication” to ensure scientific results can be reproduced, including studies that were not publicly funded.
2. Implement new post-market surveillance programs at the FDA and the National Institutes of Health (NIH) to monitor the safety of pediatric drugs.


3. Expand initiatives linking data from electronic health records, medical claims and other inputs to inform studies of chronic diseases.
4. Create a federal task force to streamline the use of federal datasets using artificial intelligence (AI) and machine learning technologies.
5. Reform the generally recognized as
(CONTINUED ON PAGE 14)

By anSLey FranCo
The headlines were designed to alarm: CT scans may account for five percent of all future cancer diagnoses in the U.S. annually. After the release of a JAMA-published study and its circulation by PBS and other outlets this spring, patients started quoting those numbers back to their physicians in exam rooms across the country.
But according to Birmingham neurosurgeon Phillip Cem Cezayirli, MD of Haynes Neurosurgical Group, the conversation around imaging, especially head, brain and spine scans, needs a re-set.
“I’ve had people quote that paper, and they’re not quoting the actual paper, they’re quoting the headline,” Cezayirli said. “The study was all theoretical. They just looked at a few years of images and then postulated based on that.”
The national study fueling the panic noted that 93 million CT scans were performed in 2023. Abdominal and pelvic CT scans contributed to the largest share of projected cancers with nearly 40 percent.
But missing from the broader coverage, Cezayirli says, is critical context: for

Phillip Cem Cezayirli, MD
neurological conditions, imaging often leads to earlier diagnosis, faster treatment and better outcomes even when the symptoms are as commonplace as a headache.
Cezayirli’s approach to imaging in neurosurgery follows a clear principle: If there are any red flags, you get imaging. Whether it’s a new onset headache, unexplained neurological symptoms, or persistent spine pain, imaging isn’t just a diagnostic tool, it’s a form of reassurance and risk reduction.
“The best way to look at it for me is: are you going to regret not getting the imaging more than regret getting the



Rowe, Dr. OT, OTR/L, FAOTA is the Co-Director of the Tourette Syndrome Center of Excellence at Children’s of Alabama, which is one of only 23 such clinics in
imaging?” Cezayirli said. “If you have no symptoms, you don’t need to get images. I think if someone has symptoms or signs of disease, better to get imaging and know that it’s nothing that needs any type of treatment than to continue to wonder.”
That perspective stems in part from his commitment to value-based care. While imaging may seem like an unnecessary cost to some, it often prevents far more expensive, or irreversible, complications later.
Not all imaging is equal. “CT is typically the first line for neurological issues due to its speed, availability and affordability,” Cezayirli said. “The entire process of conducting a CT scan takes less than five minutes to complete, with the actual imaging taking less than a minute. And at an independent facility, it’s about $100.”
An MRI, on the other hand, is more sensitive and detailed, but significantly more expensive, often costing several thousand dollars, and takes up to 30 minutes per scan. While it’s the preferred modality for evaluating soft tissue or chronic neurological conditions, it’s not always practical as a first step.
For patients who are pregnant or of childbearing age, CT is used more cautiously due to the radiation exposure. In these cases, MRI or alternative strategies may be employed, in line with the “As Low As Reasonably Achievable” (ALARA) principle established by the American College of Radiology more than a decade ago.
Cezayirli is the first to acknowledge that radiation exposure is a real consideration. But the risks are routinely overstated, he says, particularly when they’re presented without clinical nuance.
“I am annoyed that JAMA would publish a paper like that. PBS probably should’ve been more responsible in knowing that a headline like that is completely misleading,” Cezayirli said. “
He emphasizes that patients should advocate for themselves and ask informed questions, but not let headlines dictate their care. Rather than discouraging imaging, Cezayirli believes the conversation should shift toward responsible use and better education.




By Steve SPenCer
As a non-profit, Hudson Alpha (HA), located in Huntsville, is a global leader in biotechnology and genomic research. HA scientists have made discoveries in ALS, childhood genetic disorders, and kidney cancer. They’ve expanded research in bipolar and schizophrenia and are conducting ongoing investigations in a number of disease states, including cancer, Parkinson’s, lupus, and multiple sclerosis.
In an effort to put their expertise to use for the general public, in 2015 Hudson Alpha founded the Smith Family Clinic for Genomic Medicine. “The clinic opened with the idea to be the first stand-alone genomics clinic in the nation,” said Meagan Cochran, MS, CGC, the Director of The Smith Family Clinic. “We wanted to focus on genomics as this idea behind solving undiagnosed cases of suspected genetic disease.”

The clinic provides genetic counseling to patients with an existing diagnosis or family history of disease; administers tests for women who plan to become pregnant; But their largest patient group is made up of people who are battling a disease without a diagnosis, people for whom there has been no effective treatment and no answers.
“We see everyone from babies to senior citizens, all of whom have some suspicion of an underlying genetic disease that might explain some of the symptoms they’re having,” Cochran said. “In the pediatric space, most of our patients have some sort of neurodevelopmental

differences, things like developmental delay, autism, intellectual disability, as well as children born with physical birth differences.
“And as we go on through the lifespan, things might look a little different. We see plenty of adults who were healthy until they weren’t. Maybe they developed some sort of adult onset neurological disorder or cardiac condition. But something about it has triggered their healthcare team to wonder if this could have a genetic cause. And that’s how they get to us.”
A clinical geneticist and/or a genetic counselor will see the patient, and evalu-
ate their symptoms to determine what genetic test is appropriate. “Sometimes we’ll only need to order a relatively simple genetic test, maybe taking a closer look at their chromosomes or doing a set of genes that are all connected to one particular type of disorder,” Cochran said. “But there are other times when we see a person, and we’re not really sure what it is. In that case, we might go wide and do genome sequencing or reading through all of the DNA to try to get to an answer.”
While there are many genetic variants in which the symptoms are obvious from birth, researchers are still working to understand how a person can be healthy until a disease emerges later in life, even though the DNA difference was there the whole time.
“A person can be born with some level of genetic risk for a given disease,” Cochran said. “It’s not due to any one particular gene. It may be a combination of variants which you can’t change. But you can change things like your lifestyle. Imagine a jar that you have to fill up in order to have the disease. So you’re born with your jar part of the way full thanks to your DNA. But can you add or take things out of your
(CONTINUED ON PAGE 14)












When people with extraordinary talent and passion are given the technology, the facilities, and the support, they achieve great things. The discoveries and innovations happening today will help shape the future of treatments and lead to cures. And it benefits not only the patients and families who come to Children’s of Alabama, but people across the country and around the world for years to come.

continued from page 6 and comparison of rates across hospitals.
While these regulations could bring positive changes to the healthcare field for patients, they may create challenges for hospitals. The use of placeholder values in MRFs has been standard practice, and the issuance of the new CMS guidelines necessitates a shift in hospital practices concerning publication of the MRFs.
Notably, there is no specific compliance deadline included in the guidance, but hospitals should begin compliance efforts as soon as possible. Additionally,
CMS is seeking public comment on the impact of the price transparency rules through July 21, 2025. Information on submitting comments can be found at https://www.cms.gov/priorities/keyinitiatives/hospital-price-transparency/ accuracy-and-completeness-rfi.
Angie Smith is a partner in the Healthcare Practice Group at Burr & Forman. Her practice is focused on the defense of healthcare providers in medical malpractice as well as false claims litigation. She regularly counsels healthcare providers on compliance with federal and state regulations. Angie may be reached by phone at 205-458-5209 or by email at acsmith@burr.com.
Sara Petermann is a first year law school student at the University of Alabama School of Law. She spent a portion of her summer as a law clerk with Burr & Forman.
safe (GRAS) standard and fund independent studies to evaluate the safety of GRAS-affirmed ingredients.
6. Fund long-term clinical trials to compare diets in children.
7. Launch a national initiative to support studies of lifestyle interventions such as by increasing the use of randomized clinical trials into existing clinical trial frameworks.
8. Support long-term studies on the health impacts of medicines commonly prescribed to children.
9. Incorporate alternative testing models to complement animal testing in drug development and evaluation.
10. Initiate a national effort to map toxicological impacts of certain substances on childhood disease.
Based on the Report’s findings, the MAHA Commission will develop and

continued from page 10
submit a strategy to President Trump, which is expected by August. Many of the 10 steps outlined are already underway across HHS.
Continued effects of reductions in force across HHS, especially at the FDA and NIH, as well as several bills currently under consideration by Congress, will be major factors in determining whether the MAHA Commission’s goals come to fruition. The Report is anticipated to serve as a precursor to further regulatory action and enforcement, as well as future legislative activity from Congress, as many solutions suggested by the Report will require legislation to effectuate.
This article was authored by the following members of Holland & Knight’s Public Policy & Regulation Group: Senior Public Affairs Advisor Jordan Brossi, Partners Michael Werner, Meaghan Colligan Hembree and Rachel Gartner, Senior Policy Advisor Michal Freedhoff, Associates Parker Reynolds and Sara Klock, and Public Affairs Advisor Sarah Starling Crossan.
continued from page 13
jar based on your lifestyle? For example, with diabetes, things like a poor diet would add to your jar. On the other hand, good exercise would subtract from your jar.”
Even though the field of genomics has achieved extraordinary advances in the last two decades, there are still limitations. “Over the last 10 years, we’ve sequenced 2,000 people who have some sort of suspected rare disease,” said Susan Hiatt, PhD, a research faculty investigator at Hudson Alpha. “Yet, in our studies and across the United States, there are only about 50 percent who get a diagnosis. Many times, we know there is something in the genome affecting their disease, but we haven’t been able to find it. There are still a lot of genes that have not yet been described as disease genes.
“And while we think that genome sequencing is the most comprehensive test, it still has its limits, particularly where there are repetitive regions of the ge-
nome. There are a few new technologies that do longer read sequencing which are helping to identify some of these odd looking changes in the genome that we don’t understand as well yet. In fact, we just published a study in which we took a number of programs where we had previously done a short read sequencing, and we then did a long read. In about 10 percent of them, we found something that we couldn’t see before. The long read sequencing can also detect epigenetic changes which could provide a lot of good information in the future.”
If you’d like to help people who are suffering through undiagnosed genetic disease, you can donate to the Hero Fund, which was established through the HudsonAlpha Foundation to help patients who can’t afford it, get access to genomic medicine. You can find it at www. smithfamilyclinic.org/hero.

EDITOR
Steve
VICE PRESiDENT OF
Jason Irvin
CREATIVE DIRECTOR
Katy Barrett-Alley
CONTRIBUTING WRITERS
Jane Ehrhardt, Ashley Franco, Laura Freeman, Lynne Jeter, Marti Slay
Birmingham Medical News
270 Doug Baker Boulevard, Suite 700-400, 35242 205.215.7110
AD SALES: Jason Irvin, 205.249.7244
All editorial submissions should be e-mailed to: editor@birminghammedicalnews.com

Grandview Medical Center recently completed a $10 million renovation to its Women’s Services capacity.
This is the third expansion of women’s services since Grandview opened its doors in 2015. The hospital has added 10 new post-partum rooms, created a new nurses station, and added eight labor and delivery suites. Completion of the renovation brings the total number of labor
and delivery suites to 26 and postpartum rooms to 47.
“Last year, Grandview helped welcome more than 3,300 babies,” said Daniel McKinney, CEO at Grandview Medical Center. Grandview offers a full scope of services for expectant mothers, including childbirth classes, a well-baby nursery, Level III Neonatal Intensive Care Unit, and Obstetric Emergency Department.
The Alabama Healthcare Hall of Fame (AHHOF) is seeking nominations for the Class of 2026. Founded in 1997, AHHOF honorees represent a wide range of healthcare professionals, including physicians, nurses, dentists, pharmacists, scientists, administrators, educators and other related fields.
The deadline for nominations is December 15, 2025. The nominee’s contributions must distinguish him or her from the mainstream of others working in the field. The criteria for nominating an individual can be found on the AHHOF website at healthcarehof.org.
Laura Grill, CEO of East Alabama Health (EAH), has been included on the 2025 “Women Hospital Presidents and CEOs to Know” list from Becker’s Hospital Review.
Under Grill, EAH has opened the Spencer Cancer Center and Auburn Medical Pavilion, launched construction on a new center for mental health, began a $43 million critical care expansion at East Alabama Medical Center, and earned national recognition for clinical excellence in stroke, robotic surgery and diabetes care.

Grill was also appointed by Gov. Ivey to the board of the new Alabama School of Healthcare Sciences, and she completed a one-year term as chair of the Alabama Hospital Association.
Grill is one of only three CEOs from Alabama to appear on the list.

birminghammedicalnews.com
Nominations can be emailed to cmitchell@nolandhealth.com or mailed to Alabama Healthcare Hall of Fame, 600 Corporate Parkway, Suite 125, Birmingham, AL 35242. You may also direct questions to Carol Mitchell at (205) 783-8484.

Jeremy Lindley, MD has been named Chief Medical Officer (CMO) at UAB St. Vincent’s Birmingham. Lindley has been an Emergency Medicine physician at UAB St. Vincent's Birmingham since 2007. He has served on the Medical Executive Committee since 2009 and has held various leadership positions, including Chairman of Ethics Committee, UAB St. Vincent's Birmingham ED Medical Director, and System Medical Director for both Emergency Medicine and Hospital Medicine.
Lindley has also served as Board Chair for the Birmingham Regional Emergency Medical Services System (BREMSS). He is passionate about performance improvement, decreasing variation in practice, adoption of best practice guidelines, patient safety, and quality.













practice structure statement - criadv.com/disclaimer

With the nation’s largest healthcare law practice according to Modern Healthcare, Holland & Knight has more than 400 experienced attorneys covering virtually every segment of the healthcare industry, from transactional matters, regulatory compliance and real estate to litigation, government enforcement and public policy issues.
Our dedicated healthcare attorneys and professionals – in Birmingham and throughout the country – have the insight, experience, depth and resources to help promote and protect your interests.