7 minute read
DNA Properties
prokaryotic cells. There is some DNA in the mitochondria of eukaryotic animal cells and some DNA in the chloroplasts of eukaryotic plant cells.
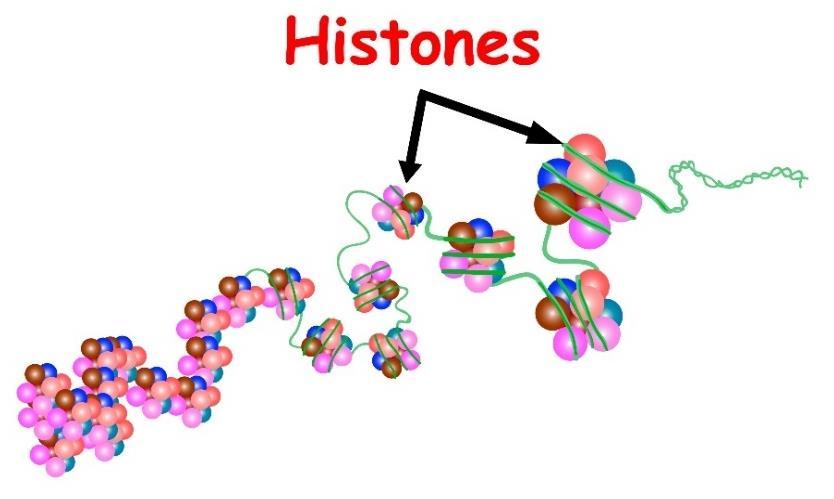
Histones are the proteins that compact the chromosomes so they fit into the small nucleus. Histones are small proteins that intertwine between the DNA strands, allowing them to stack better to make a smaller, thicker, and shorter strand. Prokaryotes generally do not have histones. Histones may have some role in the transcription of DNA, deciding which ones will open up for transcription and which ones will not be transcribed.
Advertisement
Figure 3 shows histone proteins attached to a DNA molecule:
Figure 3
As mentioned, DNA is a long polymer made from repeating nucleotides. It is extremely long and is capable of coiling into loops and other shapes to condense it within the nucleus. In all species, DNA is double-stranded, consisting of two helical strands that are bound by hydrogen bonding. Each chain is coiled around the same axis. The largest human chromosome is chromosome 1, which contains about 220 million base pairs of nucleotides. If straightened, it would be about eighty-five millimeters in length.
DNA is not a single molecule but is two separate long strands of nucleotides that are connected in a double helix. The backbone of the DNA is strong, consisting of covalent bonds of alternating nucleotide bases and phosphate groups. The nucleobase that makes up the backbone of the molecule is a nucleoside (a sugar group) and a phosphate group, which makes it a nucleotide. Many nucleotides connected to one another is called a polynucleotide, which is the type of molecule a DNA molecule is.
The type of sugar found in DNA is 2-deoxyribose, which is a 5-carbon pentose sugar. They are joined together by phosphate groups to make phosphodiester bonding between the third and fifth carbon atom of adjacent sugar groups. These bonds are asymmetric, which give directionality to the DNA strand. It runs from a 5’ carbon end to a 3’ carbon end, with the 5’ end having a terminal phosphate group and the 3’ end having a terminal hydroxyl group. In RNA, on the contrary, ribose instead of 2-deoxyribose is the sugar in the molecule.
The DNA double helix is stabilized by two separate forces. The first is the hydrogen bond between the two nucleotides and the second is base-stacking that occurs between the aromatic nucleotides. In the aqueous environment that DNA resides in, the bonding between the nucleotide bases is directly perpendicular to the axis of the DNA molecule so they aren’t in close contact with an aqueous environment. As mentioned, adenine always hydrogen-bonds with thymine, while guanine always hydrogen-bonds with cytosine.
There are two classifications of nucleotides. There are those based on purine units. These are adenine and guanine. There are also pyrimidines, which are the cytosine and thymine molecules. Another pyrimidine is the uracil molecule found on RNA. In general, a purine always connects to a pyrimidine, and vice versa. Uracil in RNA can be found in the DNA of certain bacterial species but this is a rare occurrence. A few microorganisms have a base called Base J which is beta-d-glucopyranosyloxymethyluracil instead of uracil, but this is also rare. It is simply a modified form of uracil. Base J appears to be a termination signal for RNA polymerase in these organisms, which includes species of Euglena and Diplonema.
Figure 4 identifies the purines and pyrimidines seen in DNA and RNA:
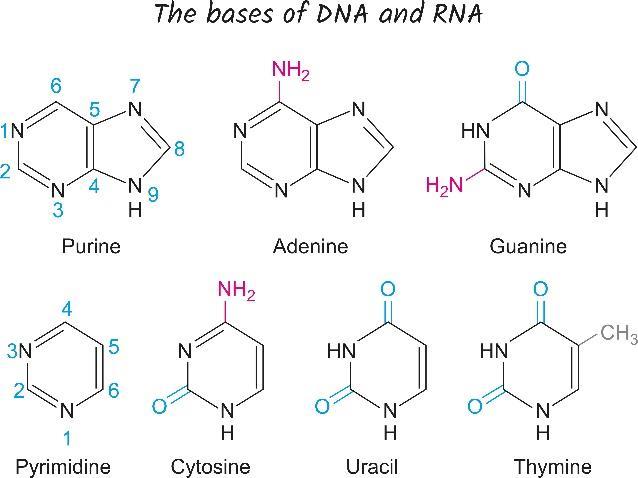
Figure 4
There are grooves that form because the DNA forms a double helix. The grooves may be where the mRNA binds to make the nucleotide by transcription but this is not known for certain. There is a wide major groove and a narrower minor groove. Because the grooves provide for a wider separation of nucleotides, this may be where transcription factors can bind to allow transcription to happen along a strand of DNA.
Base pairing between nucleobases happens by means of hydrogen bonding. Always, it is the purine that interacts with the pyrimidine. There are two hydrogen bonds between adenine and thymine and three hydrogen bonds between guanosine and cytosine, which makes the guanosine-cytosine pairing more stable but not as strong as a covalent bond. These weaker hydrogen bonds are not very strong so the strands can easily separate to allow the DNA to be replicated or transcribed as part of the duplication process. The fact that the two strands are identical, even though they are antiparallel, ensures that the information is not lost, even if there is a mutation.
The actual structure of the double-stranded DNA molecule depends on the intrastrand base stacking, which is different, depending on which bases are stacked one upon another. The strongest part of the DNA molecule is when G-C pairs stack one upon the other, with the weakest part of the DNA happening when the A-T pairs stack one upon the other on the same strand. When the two strands separate, this is called melting and forms two separate singlestranded pieces of DNA or ssDNA. This can occur at different pH extremes and secondary to high temperature. Low salt concentrations also favor the separation of DNA strands.
Longer stretches of DNA are more stable than shorter stretches of DNA. The melting temperature, which Is the temperature at which half the double-stranded DNA molecules are melted into single-stranded molecules varies according to the ionic strength and concentration of DNA in a solution. This means that those DNA strands with more GS stacking and a high overall length will melt at higher temperatures than shorter strands with more AT stacking. There are some promotor strands, labeled TATAAT, which are weakly connected, that favor the starting of DNA separation when the strand of DNA needs to be transcribed according to the normal transcription process.
The actual melting temperature of DNA is only important in laboratory settings when DNA has to be separated for evaluation purposes. The actual temperature of DNA in a living cell is basically the same all the time so separation happens under other circumstances besides an actual change in the temperature of the cell.
The DNA sequence is called a “sense” sequence if it is the sequence used to copy the messenger RNA segment and is called an “antisense” sequence if it is the opposite sequence
and is not coded. Both sense and antisense sequences can exist on the same piece of DNA so there isn’t just one strand of DNA that contains all the encoded segments.
There are a few DNA sequences that are overlapping, with segments that are contained on the same strand of DNA that are coding for two separate proteins and segments that are both sense and antisense strands. They code for one protein when coded for by one strand and code for another protein when coded for by another strand. This is more common in bacteria and viruses than in eukaryotes. Supercoiling happens to DNA segments. When DNA is “relaxed”, one strand circles the axis of the double helix every 10.4 base pairs. When it is supercoiled, it is more tightly coiled so that more bases are encompassed in a turn of the helix. Coiling in the opposite direction, called negative supercoiling, causes the base pairs to separate more easily, making them more able to be acted upon by transcription and DNA replication.
DNA consists in different conformations, including A-DNA, B-DNA, and Z-DNA, although most organisms only have B-DNA and Z-DNA. Each conformation of DNA differs in the type and concentration of the amount of metal ions on the DNA and the presence of polyamines in solution. B-DNA is the conformation of DNA found in most cells. The A-DNA isn’t found in functional cells and has a wider right-handed spiral, with a shallow minor groove and a narrow, deeper major groove. It only occurs when DNA is partially dehydrated so it isn’t found under normal situations.
At the ends of the DNA strand, there are segments of DNA called telomeres. The main function is to allow the cell of replication the ends of the chromosome using an enzyme known as telomerase because the normal replication enzymes can’t operate at the extreme ends of the 3’ part of the strands so telomerase must do the replication process. Telomeres are a way to cap the 3’ end of the strand, protecting the end of the strand from not being replicated. Telomeres in humans are repeated segments labeled TTAGGG, which are recognized by telomerase.
Because telomeres contain a lot of guanine, they stabilize the ends of the chromosome because they stack in a more stable way than other nucleotides. Four guanine molecules stack in a flat plate, one on top of the other, to form what is known as a G-quadruplex structure that is very stable and allows for chelation of metal irons in the middle of each of the four-base units. Telomeres also form large loops called T-loops or telomere loops. In these loops, singlestranded DNA is able to curl around in a long circle that is stabilized by telomere-binding proteins. At the extreme end, a triple-stranded loop is made, called a displacement loop or a Dloop.
Chromosomes are packed in a structure called “chromatin”. Base modifications are involved in the packaging of DNA, with non-coding regions being high in methylated base pairs or in areas




