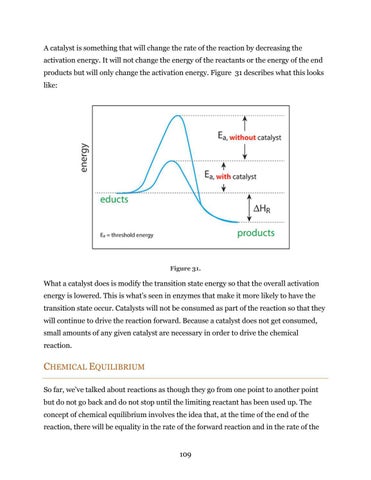
A catalyst is something that will change the rate of the reaction by decreasing the activation energy. It will not change the energy of the reactants or the energy of the end products but will only change the activation energy. Figure 31 describes what this looks like:
Figure 31.
What a catalyst does is modify the transition state energy so that the overall activation energy is lowered. This is what’s seen in enzymes that make it more likely to have the transition state occur. Catalysts will not be consumed as part of the reaction so that they will continue to drive the reaction forward. Because a catalyst does not get consumed, small amounts of any given catalyst are necessary in order to drive the chemical reaction.
CHEMICAL EQUILIBRIUM So far, we’ve talked about reactions as though they go from one point to another point but do not go back and do not stop until the limiting reactant has been used up. The concept of chemical equilibrium involves the idea that, at the time of the end of the reaction, there will be equality in the rate of the forward reaction and in the rate of the
109