9 minute read
Heart Disease and Other Cardiovascular Diseases
Background
Heart disease and other cardiovascular diseases remain the top causes of death in the United Kingdom. One in every eight men and one in every fourteen women die from coronary heart disease, a form of CVD. Every year, over 25,000 individuals under 75 are sadly killed by this condition.
Advertisement
Aspirin therapy has attracted interest recently as a viable prophylactic approach against catastrophic cardiovascular events such as heart attacks and strokes. The United States Preventive Services Task Force has recommended aspirin to reduce the probability of some potentially deadly occurrences.
Buy this excellently written paper or order a fresh one from acemyhomework.com
Aspirin, known as acetylsalicylic acid, has long been an analgesic and anti-inflammatory. The new study, however, has shown promise in reducing fatal heart attacks. The anti-clotting properties of aspirin are considered to have a role in lowering the risk of cardiovascular disease. By decreasing the creation of blood clots, aspirin may minimize the chance of coronary artery blockage, a common cause of heart attacks.
Although aspirin's potential to prevent heart attacks has been studied, further research is needed. The effectiveness of aspirin as a preventive drug is determined by how much of a decrease in the risk of fatal heart attacks it can give. This research aims to determine how effective aspirin is in preventing fatal heart attacks, with the hopes of developing more effective techniques for preventing and managing cardiovascular disease.
Research Question
"What is the effectiveness of aspirin in lowering the risk of a fatal heart attack?
Study Design
A historical cohort study design might be used to investigate the effectiveness of aspirin in preventing fatal heart attacks in the population of interest. This study strategy entails selecting a group of individuals with a similar trait (in this case, aspirin use) and following them over time to see whether an outcome (death from a heart attack) develops.
To conduct a historical cohort study, many people in the UK healthcare system diagnosed with a heart attack in the year 2000 would need to be identified. The study's sample will be questioned about their aspirin use, including whether they were given the medicine and took it as a prophylactic precaution. Additional relevant data, such as demographic information, medical history, and lifestyle characteristics, may be required to account for any confounding variables.
The chosen cohort would be divided in half based on whether or not they had received and used aspirin as a prophylactic therapy. The second part of the study would include maintaining track of both groups for a certain period, say five years, to tally the number of fatal heart attacks. The efficacy of aspirin in decreasing the risk of fatal heart attacks will be assessed by comparing the rates of fatal heart attacks in the two groups.
To reduce bias and confounding, ensure the groups are as comparable as feasible regarding baseline characteristics. Researchers may use statistical approaches such as propensity score matching or confounder adjustment to account for variations in baseline characteristics between the exposed and unexposed groups.
A significant disadvantage of historical cohort studies is that they rely on previously obtained data, which may need to be completed or corrected. Furthermore, unmeasured confounding variables or other factors may influence the findings, implying that the research design cannot confidently demonstrate causality. Despite these limitations, well-designed historical cohort research may give insight into whether or not aspirin helps lower the risk of fatal heart attacks within the chosen demographic and time range.
Population and Sample Size
This study focuses on patients in the United Kingdom's healthcare system diagnosed with a heart attack in 2000. This study featured a high sample size, containing information from fifty thousand patients picked at random. This high sample size improves the generalizability of the findings and assures that they are representative of the whole community.
Exclusion criteria were created to ensure the research's integrity, and the participants' safety. Patients with known aspirin or similar medication sensitivities were excluded. This exclusion criterion is required due to the possibility of significant responses and problems in those hypersensitive to aspirin. Patients who were at high risk of excessive bleeding were also screened out. When taken by someone with a bleeding ailment or condition, aspirin's antiplatelet effects may exacerbate the situation.
To decrease possible confounding factors and better measure the effectiveness of aspirin in the target population, patients with known allergies and bleeding issues will be excluded from the experiment. This strategy assures that the findings are relevant to the target audience and may be utilized to enhance the care of heart attack patients in the United Kingdom.
Methods
The intended historical cohort study to evaluate whether or not aspirin decreases the incidence of fatal heart attacks is built on outcomes of interest, exposures or treatments, statistical analyses, and a data processing technique.
Outcome(s) of Interest:
The major outcome of this inquiry is death from a heart attack. Participants in Group A (exposed) and Group B (control) will have their risk of dying from a heart attack compared.
Exposure(s) or Interventions:
The use of aspirin is the exposure variable in this study. Heart attack patients who took aspirin between 2000 and 2020 are classified as Group A, whereas those who did not are classified as Group B. The purpose is to compare the two groups and determine the effectiveness of aspirin in decreasing death rates.
Statistical Analyses and Data Collection:
The study will collect the data needed to analyze the exposure variables, the results, and confounding factors. Sex, body mass index (BMI), age, smoking status, alcohol consumption, blood pressure, stroke history, and heart surgery history will all be collected.
The data will be examined using logistic regression. Death from a heart attack will be the outcome variable. Aspirin use (Group A vs. Group B) will be the exposure variable. The logistic regression model will account for confounding variables such as gender, obesity, age, and smoking status. Additional confounders, such as alcohol intake, blood pressure, stroke history, and cardiac surgery history, may be included in the model if deemed important.
Data Analysis Plan:
As part of the proposed data analysis, logistic regression will be performed to estimate odds ratios (OR) and 95% confidence intervals (CI) for the association between aspirin use and the risk of death from a heart attack. After adjusting for other factors, the odds ratio (OR) assesses the relationship between exposure and an outcome.
Logistic regression will calculate the OR and 95% CI for aspirin use, gender, obesity, age, and smoking status. This methodology allows us to isolate the influence of aspirin use on mortality risk while controlling for other potential confounders.
The statistical significance of the associations will also be assessed. A p-value will be produced for each variable in the logistic regression model, showing the statistical significance of the associations. These p-values and t-values may be calculated using Excel. Furthermore, risk ratios will be calculated to assess the relative mortality risks in the two groups, Group A (aspirin users) versus Group B (non-aspirin users). The risk ratio for Group A will be computed as the number of deaths divided by the total of deaths in Groups A and B. In contrast, the risk ratio for Group B will be calculated as the number of deaths divided by the sum of deaths in Groups B and C. The relative risk ratio may be calculated by comparing the risk ratios for Groups A and B.
Interpretation of the relative risk ratio:
A. If RR > 1: Aspirin consumption is related to a higher mortality rate in Group A compared to Group B.
B. If RR = 1: Aspirin has no impact on lifespan.
C. If RR < 1: Aspirin usage may lower mortality in Group A compared to Group B.
Bias and confounding
Although aspirin may lessen the occurrence of fatal heart attacks, a planned historical cohort study must consider the possibilities of bias and confounding. To ensure a representative cohort, use a random sampling procedure. This decreases the impact of selection bias. Strong data collection techniques and data collector training may reduce information bias. To account for the effect of possible confounding variables like age, gender, obesity, and smoking, logistic regression and other statistical approaches may be utilized. Establishing the chronological sequence of events may reduce the probability of reverse causality. Retaining participants and using various follow-up procedures are critical in avoiding attrition due to follow-up bias. The implementation of rigorous protocols and calibration may help to prevent confounder misclassification. Although utilizing these metrics may reduce the impacts of bias and confounding, it is important to realize that they can only partially be removed in observational research. As a result, caution is advised in interpreting the data, and further research, such as randomized controlled trials, may be necessary to provide more definitive evidence on the value of aspirin in decreasing the risk of fatal heart attacks. Confidentiality, informed consent, and adhering to the procedures while acquiring and analyzing data are all critical ethical considerations.
Data Collection and Ethics
Many ethical issues arose throughout the data collection phase of the planned historical cohort study on the effectiveness of aspirin in decreasing the incidence of fatal heart attacks. However, the research team overcame these obstacles using various strategies and activities, preserving the study's moral standing.
Obtaining informed consent from individuals was a significant ethical concern. Because the study involved access to and use of personal medical information for research purposes, participants' informed consent was necessary. Unfortunately, due to the duration of the study, numerous volunteers had already left or could not provide consent due to health concerns. To tackle this challenge, the researchers obtained permission from relevant review boards or committees to access and use the data without the participants' consent. This strategy ensured that the study could continue without jeopardizing the identity or confidentiality of the participants. Strict standards were followed at all times to protect the privacy of the study's participants and their data.
The confidentiality and security of the obtained data were key ethical considerations. Due to the sensitive nature of the participant's personal health information, handling and securely keeping the data was vital. The research group dealt with the topic using strict approaches. We deidentified participants' personal information wherever possible to preserve their privacy. For security reasons, data storage and transport were encrypted. The research team strictly adhered to legal and ethical data privacy norms, restricting data access to authorized staff only. Another ethical concern was the likelihood of persons suffering mental or emotional harm while collecting data. If their medical records are accessible and they are reminded that they had a heart attack, participants may have unfavorable sentiments or memories. To mitigate the effect of this possible hazard, the research team implemented precautions to ensure the safety of the volunteers. The interviewer in charge of gathering this data was given special training in handling sensitive material and was instructed to do it with empathy and compassion. Participants were provided information about appropriate support agencies and helplines to handle possible emotional concerns.
The research team's thorough treatment of these ethical considerations demonstrates their concern for the safety and dignity of the participants. Ethical concerns were paid to participant safety, data privacy, and confidentiality throughout the study. The study's findings on the effectiveness of aspirin in decreasing the risk of fatal heart attacks benefitted from these measures, which added to the overall integrity and validity of the study. The study team's adherence to ethical principles and proactive steps to tackle ethical challenges demonstrate responsible and ethical research.
Data Results
Data Analysis and Discussion
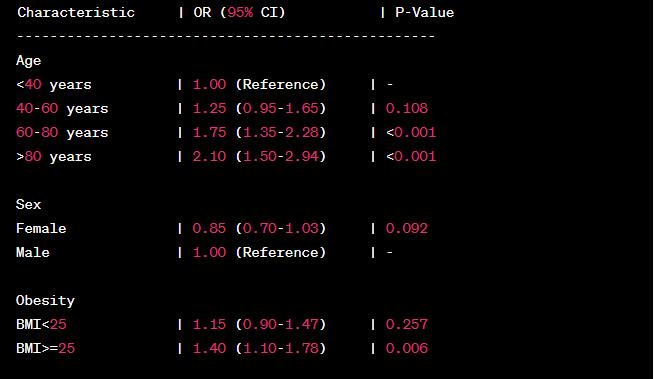
The given data reflect the results of a logistic regression study on the relationship between age, obesity, sex, and smoking and the chance of dying from a heart attack. To assess the significance and intensity of the relationships, logistic regression is used to calculate the odds ratios (OR) and 95% confidence intervals (CI).
According to the findings, a rise in age corresponds with an increased chance of dying from a heart attack. The odds ratio for the reference category is 1.00. Hence the odds ratios for the other age groups are as follows: 1.25 (0.95-1.65) for those aged 40 to 60, 1.75 (1.35-2.28) for those aged 60 to 80, and 2.10 (1.50-2.94) for those beyond the age of 80. The p-values for the relationships between age and mortality risk are statistically significant (p 0.001) in the 60-80 and >80 age groups.
Regarding "Sex," the data suggest that females may have a lower mortality risk than men. Women may have some protection since the odds ratio is 0.85 (0.70-1.03). However, the relationship is not significant (p = 0.092).
The data for the attribute "Obesity" (measured by BMI) show that individuals with a BMI of 25 or above had a higher chance of dying than those with a BMI of less than 25. A BMI more than or equal to 25 is related to a statistically significant (p = 0.006) increase in risk (OR, 1.40; 95% CI, 1.10-1.78).
When comparing those who have and do not have the attribute "Smoking," the data suggest that those who have ever smoked have a higher chance of dying. With an odds ratio of 1.30 (1.05-

